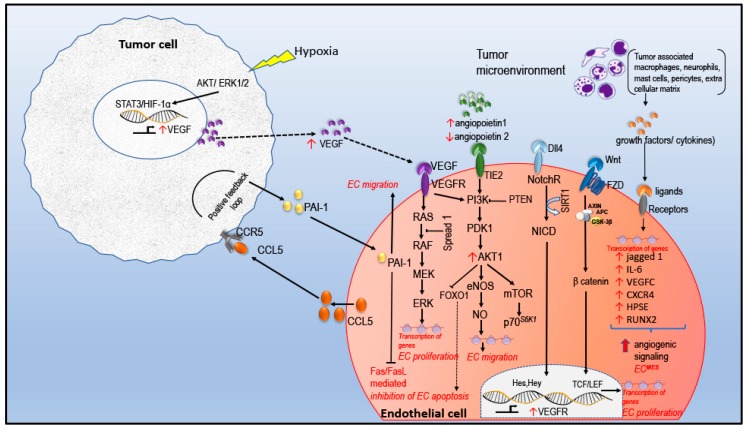
Figure 1.
Illustration of tumor endothelial cell signaling. In tumor micro environment (TME), angiogenesis is mainly triggered by hypoxia which promotes generation of pro-angiogenic factors such as growth factors and cytokines by tumor cells and tumor associated stromal cells. Vascular endothelial growth factor/vascular endothelial growth factor receptor (VEGF/VEGFR) is the main axis of angiogenesis and hence is the most attractive target for anti-angiogenic treatment in cancer therapy. In invasive breast cancer (BC), VEGFR3 is up-regulated in tumor endothelial cell. Under low oxygen tension, transcription of HIF-1 α is increased which increases the synthesis of stress related proteins such as VEGF by tumor cells. Binding of factors to the endothelial cell (EC) receptors activates angiogenic signaling pathways mainly PI3K/AKT/mTOR/eNOS signaling. C-C motif chemokine ligand 5 (CCL5) a member of the cytokine family is detected in tumor samples. Increased plasminogen activator inhibitor-1 (PAI-1) secretion by the tumor cell up-regulates CCL5/CCR5 axis forming a +ve feedback loop leading to increased expression of transcription factors related to epithelial–mesenchymal transition (EMT). In addition, PAI-1 protects EC cells from Fas/Fas ligand (FasL) mediated apoptosis. Wnt signaling regulate angiogenesis via β catenin, as a result transcription factors TCF/LEF bind to promotor region of Wnt transcribed genes leading to EC cell proliferation and morphogenesis. The EC cells acquire mesenchymal phenotype in a TME, showing increased migratory, invasive and angiogenic property. Sustained EC cell signaling activates angiogenic process including proliferation, inhibition of apoptosis, migration, ultimately building the tumor vasculature [10,11,12].