Abstract
The multidomain protein encoded by the Tumor Susceptibility Gene 101 (TSG101) is ubiquitously expressed and is suggested to function in diverse intracellular processes. In this review, we provide a succinct overview of the main structural features of the protein and their suggested roles in molecular and cellular functions. We then summarize, in more detail, key findings from studies using genetically engineered animal models that demonstrate essential functions of TSG101 in cell proliferation and survival, normal tissue homeostasis, and tumorigenesis. Despite studies on cell lines that provide insight into the molecular underpinnings by which TSG101 might function as a negative growth regulator, a biologically significant role of TSG101 as a tumor suppressor has yet to be confirmed using genuine in vivo cancer models. More recent observations from several cancer research teams suggest that TSG101 might function as an oncoprotein. A potential role of post-translational mechanisms that control the expression of the TSG101 protein in cancer is being discussed. In the final section of the review, we summarize critical issues that need to be addressed to gain a better understanding of biologically significant roles of TSG101 in cancer.
Keywords: TSG101 protein, cell death, cell survival, gene deletion, knockout, mouse, mutagenesis, oncogenes, transgenes
1. Identification of the Tumor Susceptibility Gene 101 (Tsg101)
In the mid-1990s, the laboratory of Stanley Cohen at Stanford University conducted an insertional mutagenesis screen on immortalized mouse embryonic fibroblasts (NIH3T3 cells) to identify candidate tumor suppressor genes [1]. The functional knockout of both copies of a targeted locus was achieved through the expression of antisense transcripts from a retroviral gene search vector in an LAP348 transactivator-mediated manner. Using this approach, Li and Cohen identified more than 20 clones that exhibited anchorage-independent growth potential in soft agar, and one of these clones, called SL6, was expanded into a cell line. The transformed phenotype of these cells could be reversed when the expression of the antisense transcript was terminated through Cre recombinase-mediated excision of the transactivator transgene. Following identification of the insertion site of the gene search vector in SL6 cells and cloning of the extended cDNA from an expression library, the authors named the transcript originating from the targeted locus Tumor Susceptibility Gene 101 (Tsg101). Designating this gene as a TSG seemed appropriate at the time since the exogenous expression of the Tsg101 cDNA in both antisense and sense orientation resulted in a transformed phenotype. In 1997, TSG101 had become more widely known as a tumor suppressor when it was reported that this gene is mutated, or its expression is lost in a significant subset of sporadic breast cancers [2]. These findings could not be confirmed [3,4,5,6], and it was also demonstrated later using immunoblot that ‘TSG101-deficient’ SL6 cells still express the TSG101 protein [7]. Regardless, TSG101 is still assigned as a tumor suppressor or negative growth regulator in online NCBI Resources and other internet databases.
2. TSG101 is a Housekeeping Gene
The coding sequences of the mouse and human TSG101 mRNAs are 86% identical on the nucleotide level [2]. Sanger sequencing data generated by our team revealed that the genomic organization of the Tsg101 locus is highly conserved between mouse and human [8], and the mRNA transcripts span across 10 exons and not six as previously reported. The revision of the genomic structure of TSG101 implied that the majority of truncated transcripts that were observed in cancer and nonmalignant tissues are true alternative splice products that originate from exon skipping, rather than aberrant transcripts from cryptic splice sites as proposed earlier [2,9,10,11]. The genomic sequencing results also demonstrated that the translation of the protein starts precisely with the known Kozak consensus motif [12]. The 5′ region preceding the first exon is typical for housekeeping gene promoters as it lacks TATA and CAAT boxes, and the highly GC-rich sequence contains several consensus sites for Sp1, AP2, and GAPBF2 [8]. In support of this notion, TSG101 is expressed in all tissues and cell types [2,8], and the analysis of expressed sequence tags (ESTs) revealed that the Tsg101 mRNA is already present in 1-cell and 2-cell stage mouse embryos. The expression of Tsg101 in germ and stem cells may also explain the origin of a processed Tsg101 pseudogene in the mouse genome, which made it challenging to identify the actual Tsg101 locus and isolate genomic DNA sequences for the construction of gene targeting vectors to generate knockout mice [8,13,14,15]. Despite ubiquitous expression in all tissues, it might be worth noting that the highest Tsg101 mRNA levels were observed in the brain and the lactating mammary gland [8].
The designation of Tsg101 as a housekeeping gene had several implications. First, a complete knockout of Tsg101 might cause early embryonic lethality [8]. More importantly, significant variations in high or low protein expression levels in normal tissues or cancer cells are likely a consequence of post-transcriptional or post-translational mechanisms. In the postgenomic era, this is an important fact to consider since mRNA expression levels from microarray and RNA-sequencing data are frequently being used to judge the importance of genes in cancer development and patient survival. As discussed later in this review, a tight post-translational control of the TSG101 protein level that balances variations in mRNA expression also imposes challenges for the generation of genetically engineered models to assess the effects of TSG101 gain- or loss-of-function in normal organogenesis and cancer development.
3. TSG101 Encodes a Multidomain Protein
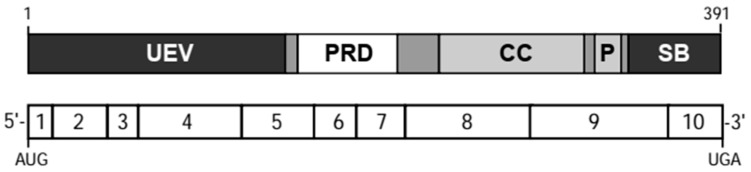
The human and mouse TSG101 transcripts encode proteins of approximately 50 kDa in size with more than 94% similarity. The TSG101 protein contains several conserved domains (Figure 1). The N-terminal region is a ubiquitin-conjugating enzyme E2 variant (UEV) domain, which has been shown to bind ubiquitin but it lacks enzymatic activity due to the absence of the active site cysteine residue that is required for the transfer of ubiquitin [16,17,18,19]. Based on this unique structural feature, TSG101 was suggested to function as a negative regulator of ubiquitin-mediated protein degradation [16] as well as a mediator for the intracellular movement of ubiquitinated proteins [19]. The UEV domain also contains a hydrophobic groove that facilitates the association of TSG101 with polypeptides that contain specific PTAP or PSAP amino acid motifs such as regulatory proteins for intracellular trafficking and retroviral proteins [20,21,22,23]. Other conserved structural features of TSG101 are a proline-rich region (PRD) that is typically found in surface proteins and transcription factors [2] as well as a coiled-coil (CC) domain that has been shown to interact with stathmin [24]. The C- terminal end of TSG101 was designated as a ‘steadiness box (SB)’ because of its critical role for the post-translational autoregulation of steady-state levels of the TSG101 protein [25]. Interestingly, TSG101 contains an intrinsic PTAP amino acid motif located between the CC and SB that might modulate the binding of proteins to the N-terminal UEV domain of TSG101 [26].
Figure 1.
Schematic of the functional domains of the mouse TSG101 protein and their location within the 10 coding exons of the spliced mRNA. UEV, ubiquitin-conjugating enzyme E2 variant; PRD, proline-rich domain; CC, coil-coiled domain; P, conserved PTAP tetrapeptide motif; SB, steadiness box.
4. The TSG101 Protein Mediates Diverse Intracellular Processes
On the subcellular level, most of TSG101 was observed to reside in the cytoplasm regardless of the cell cycle stage [27]. During the late S phase, a fraction of the protein can be detected in the nucleus, and TSG101 co-localizes with the spindle apparatus during mitotic cell division [5,27,28]. The temporal distribution of TSG101 within several cellular compartments is indicative that this multi-domain protein facilitates a variety of molecular and biological processes, which include the regulation of transcription [29,30], cell proliferation, and division [27,31,32], as well as the aforementioned ubiquitination and intracellular movement of other proteins.
Two reports from the laboratories of Duane Jenness and Scott Emr [33,34] were first to classify TSG101 as the mammalian ortholog of the class E Vacuolar protein sorting 23 (VPS23) in Saccharomyces cerevisiae, which is also known as STP22. Subsequent studies revealed that TSG101/VPS23 is a central member of a subset of VPS class E proteins (VPS23, VPS28, and VPS37) that form the Endosomal Sorting Complex Required for Transport I (ESCRT-I). As part of this complex, TSG101 is suggested to selectively bind ubiquitinated cargo proteins and direct their sorting into multivesicular endosomes [19]. In line with these findings, it was reported later that the downregulation of TSG101 by RNA interference causes a formation of multi-cisternal early endosomes and defects in protein sorting. These intracellular abnormalities may impair the downregulation of the endocytosed, ligand- bound Epidermal Growth Factor Receptor (EGFR) [26,35,36,37], which consequently may enhance receptor signaling in early endosomes. This suggested role may provide a molecular mechanism by which TSG101 could act as a tumor suppressor, and it was reported that ‘TSG101-deficient’ SL6 cells exhibited alterations in EGF trafficking that may have caused elevated levels of active MAP kinases following EGF stimulation [34]. Nonetheless, these early reports should be viewed with some caution since it was revealed later that the ‘TSG101-deficient’ SL6 cell line did not show any substantial reduction in the steady-state level of the TSG101 protein as determined by immunoblot [7]. More importantly, it was also demonstrated that cells completely deficient in TSG101 (i.e., genomic knockout) exhibited a significant reduction in EGFR and ERBB2 [38], and, as discussed later, there is no experimental evidence to date that would suggest that deficiency in TSG101 is sufficient to cause neoplastic transformation and cancer [7,14,15,39].
Insight into the multifaceted functions of TSG101 as a member of ESCRT-I was gained from a series of studies on the association of TSG101 with retroviral proteins and their intracellular movement [40]. TSG101 is being recruited and delivered to viral assembly sites by interacting with group-specific antigen (Gag), which has a PTAP amino acid motif in its C-terminal p6 region that can associate with the PTAP binding grove within the N-terminal UEV-domain of TSG101 [18,21,22,41,42]. The ubiquitination (Ub) of Gag permits another mode of interaction with a different region in the UEV-domain of TSG101 [23,43].
The specific functions of TSG101 in retroviral particle assembly and budding might be exemplary for a broader role in the biosynthesis and release of exosomes or, as now generally defined, secreted extracellular vesicles (EVs). Using a proteomic approach, Thery et al. [44] were first to identify the TSG101 protein within exosomes, and TSG101 is now being employed as one of the standard markers for the purification of EVs [45]. In a newer study, Colombo and coworkers [46] reported that selected ESCRT proteins modulate EV biogenesis, and the downregulation of TSG101 resulted in reduced secretion of exosomes. Since TSG101 seems to play a general role in EV assembly and release in mammalian cells and retroviruses appear to hitchhike on these EV mechanisms, it should be evident that membrane-binding determinants in the matrix domain of retroviral Gag proteins may not be the main mode of (mis)directing TSG101 and its cargoes to the cell membrane. Therefore, the specific control mechanisms by which TSG101 may traffic its ubiquitinated cargos to the cell membrane, as opposed to degradative compartments in the interior of a cell, remain to be elucidated.
In summary, the TSG101 protein is suggested to mediate a variety of intracellular processes, but the majority of published articles only highlight its proposed roles in endosomal trafficking. Notably, many of the reports that solely focus on the molecular, biochemical, and intracellular functions of TSG101 emphasize the importance of their specific findings for diseases like cancer and neurodegeneration, but the proposed mechanisms are rarely validated in genetically defined in vivo disease models or in primary human tissue samples. Consequently, the biological relevance of these multifaceted functions of TSG101 for normal development, differentiation, and tissue homeostasis, as well as potential roles of TSG101 in cancer are poorly defined. On the experimental level, most published studies that examined the intracellular roles of TSG101 relied exclusively on RNA interference, overexpression of TSG101 mutants, or the use of chemical compounds that disrupt entire cellular processes. CRISPR/Cas-based targeted knockouts or other gene editing methods to introduce mutations into the endogenous TSG101 locus have not been widely employed. As reviewed in the next section, the phenotypes of defined TSG101 genomic knockout models deviate from observations in cell lines where TSG101 is only knocked down with siRNAs. It is unclear to date which of the known molecular mechanisms, if any, are responsible for the indispensable functions of TSG101 for the proliferation and survival of mammalian cells.
5. A Knockout of TSG101 Causes Cell Cycle Arrest and Cell Death
From the analysis of the Tsg101 gene promoter sequence and expression profiling, we predicted that a conventional knockout of this gene causes embryonic lethality [8]. Indeed, Ruland and coworkers [14] were first to demonstrate that mice homozygous deficient in exons 8 and 9 fail to develop past day 6.5 of embryogenesis. TSG101 knockouts do not form a mesoderm, and they are smaller as a result of a defect in cell proliferation, which was caused by the nuclear accumulation of p53. In line with this notion, embryos that are doubly deficiency in TSG101 and p53 were able to gastrulate, but they survived for only two additional days. Our team developed a Cre/lox-based conditional knockout mouse model that allows for a temporally and spatially controlled deletion of Tsg101 in germ cells and differentiated tissues during embryonic and postnatal development as well as in cultured cells that are primary, immortalized, or tumorigenic [7,15,39]. The conditional deletion of the promoter and first exon with Cre recombinase resulted in the complete absence of the TSG101protein [7]. As controls to the genomic knockout, we examined the level of TSG101 in the ‘TSG101-deficient’ SL6 cell line described earlier and were surprised that these cells exhibited only a marginal reduction in the TSG101 protein compared to their parental NIH3T3 cells. Similar to the report by Ruland et al., we observed that a complete knockout of TSG101 causes a p53-dependent cell cycle arrest, but the deletion of p53 did not extend the survival of TSG101-deficient cells [7,39]. Re-expression of exogenous, epitope-tagged TSG101 was sufficient to restore normal proliferation and survival of cells that lacked both endogenous copies of Tsg101. More interestingly, we noted that individual rescue clones expressed exogenous TSG101 at the same levels as the endogenous protein in parental controls [7], supporting earlier reports that the amount of the TSG101 protein in a particular cell type is tightly controlled [25]. We have used this knockout-rescue approach to validate that expression of the human TSG101 cDNA can fully restore normal growth of mouse cells that lack endogenous TSG101, suggesting that both mammalian proteins function in an identical manner. In contrast to the full-length cDNA, expression of TSG101 mRNA splice variants or deletion mutants lacking individual domains of TSG101 did not rescue the deleterious knockout phenotype (Stanton and Wagner, unpublished) [47].
In contrast to mutants that lack exons 8 and 9, the Cre-mediated excision of the promoter and exon 1 of Tsg101 in the germline resulted in an earlier embryonic lethality prior to or during implantation [15]. A tissue-specific knockout of TSG101 in mammary epithelial cells of lactating female mice using Cre recombinase under control of the Whey Acidic Protein gene promoter (WAP- Cre) demonstrated that TSG101 is equally required for cell survival and tissue homeostasis in adult animals. Secretory epithelial cells that have completed their functional differentiation show a significantly reduced proliferation rate and it is therefore evident that crucial functions of TSG101 for cell survival are independent of its suggested roles in cell division. Re-expression of exogenous TSG101 in the lactating mammary gland restores normal mammary gland development and lactation [48]. Similar to the results from cell line studies, the survival of TSG101-deficient epithelial cells in the mammary gland could not be rescued by a knockout of p53 [39].
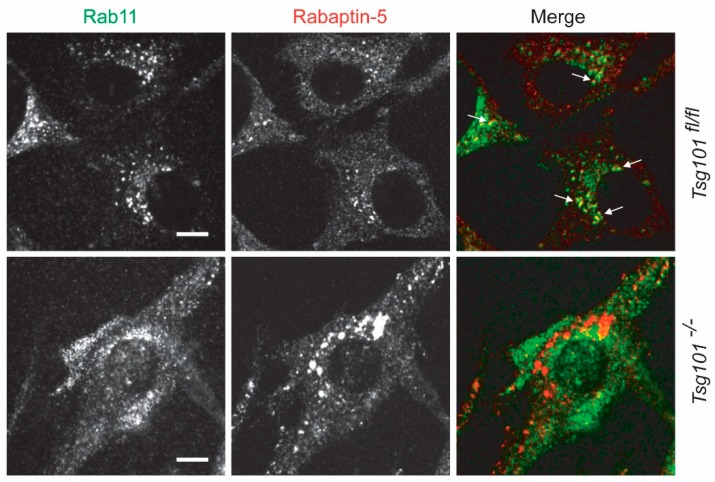
TSG101 conditional knockout mice were also utilized to examine biologically relevant functions of TSG101 for normal tissue homeostasis in organ systems other than the mammary gland. Using a cardiac-specific and tamoxifen-inducible knockout of TSG101, Essandoh et al. [49] recently demonstrated that complete deficiency in TSG101 was lethal within one week after deleting both copies of the gene in adult mice. In contrast, animals with a 50% knockdown of TSG101 through the expression of an shRNA construct were able to survive, but they exhibited defects in exercise-induced cardiac hypertrophy. This suggests that optimal expression of TSG101 is required for heart muscle growth under specific physiological conditions, and interestingly, reduced levels of TSG101 can still be lethal when the mice are being challenged with endotoxin-triggered myocardial injury [50]. On the mechanistic level, exercise-induced cardiac growth is being mediated by IGF-1R signaling through AKT, and a knockdown of TSG101 caused a defect in the recycling of the IGF1 receptor, possibly due to the downregulation of RAB11a and FIP3. These findings may support an observation from our team that shows that the knockout of TSG101 causes the dissociation of early endosomes from the recycling endosome (Figure 2). Then again, the mechanism does not explain the cell death caused by a complete deficiency in TSG101 and specifically the lethal phenotype of mice with the heart-specific knockout. In stark contrast to the TSG101 conditional knockout mice, the Cre-mediated deletion of the IGF-1R in cardiomyocytes had no noticeable effect on normal heart development [51].
Figure 2.
The knockout of TSG101 causes a dissociation of a subset of early endosomes from recycling endosomes Confocal microscopic images of TSG101 conditional knockout fibroblasts 48 h after infection with a pBabe-Cre virus (Tsg101-/-) and their isogenic control cells expressing endogenous TSG101 (Tsg101fl/fl), which were infected with the pBabe control vector. Cells were stained with antibodies against Rab11 (green) and Rabaptin-5 (red); bar represents 10 µm. Arrows indicate examples for a colocalization of both markers (yellow) in wildtype controls.
Like the mammary epithelium and cardiomyocytes, the deletion of TSG101 in oligodendroglia was reported to cause apoptosis, demyelination, vacuolation, and severe spongiform encephalopathy. Mice lacking TSG101 in parts of the central nervous system developed a tremor and had significantly reduced body weight by 8 weeks of age [52]. In a parallel study, the authors showed that conditional knockout mice deficient in the endosomal protein RAB7 did not show any histopathological abnormalities in the CNS, suggesting that the severe phenotypes associated with TSG101 deficiency are not due to any defects in late endosomal, lysosomal, or autophagolysosomal functions as proposed earlier from cell line studies. The authors concluded that the vacuolation in the brains of TSG101-deficient animals is likely a secondary consequence.
In summary, the complete loss of TSG101 triggers an accumulation of p53 and a cell cycle arrest at the G1 phase followed by cell death, which is independent of p53 function and proliferation. This finding is not unique to mammals and can be extended to other vertebrate species. A knockdown of TSG101 with antisense morpholinos causes a growth delay and lethality of zebrafish embryos [53]. On a mechanistic level, it was validated that p21Cip1 is a downstream mediator of the p53-dependent cell cycle arrest in response to the deletion of Tsg101 [39,53]. Although it was previously proposed that TSG101 controls the levels of MDM2 and vice versa [54], we demonstrated that deficiency in Cdkn2a (p16Ink4a/p19Arf knockout) did not alter the ability of MDM2 to sequester p53 and restore the p21-mediated G1 arrest. The collective results obtained from the analysis of Tsg101/p53, Tsg101/p21, and Tsg101/Cdkn2a conditional double-knockout cell lines that were generated in our team suggested that a biologically significant interaction of TSG101 with the MDM2/p53 circuit seems unlikely. The p53/p21-controlled G1 arrest might, therefore, be an indirect consequence of an activation of stress response pathways that are being triggered by the knockout of TSG101 [39].
6. TSG101 Knockout Cells Are Stressed and Undergo Autophagy Prior to Cell Death
Fibroblasts with a conditional knockout of TSG101 exhibit several characteristics of stressed cells: a) phosphorylation of mitogen-activated protein (MAP) kinases independent of growth factor stimulation, b) widespread redistribution of actin filaments, and c) induction of autophagy prior to cell death [38]. TSG101 knockout cells possess greatly enlarged lysosomes that were enriched with the autophagy-related protein LC3. Unlike previous observations in SL6 cells [34], TSG101 is not required for the routing of biosynthetic cargo from the Golgi and delivery of hydrolases to lysosomes. Cathepsin D is transported to and functional within the distended vesicles of TSG101 knockout cells [38]. While re-expression of exogenous TSG101 can revert these intracellular processes, treatment of TSG101 knockout cells with the PI3 kinase inhibitor 3-methyladenine (3MA) led to accelerated cell death, supporting the notion that TSG101 knockout cells utilize autophagy as a survival mechanism prior to their ultimate death [38].
Although the precise cellular process that causes TSG101 knockout cells to die is still unknown, one important consequence that may trigger an intense stress-response is growth factor deprivation. Unlike previous observations that a siRNA-mediated knockdown of TSG101 resulted in enhanced recycling or retention of the EGFR within early endosomes [55], the defined conditional knockout of the Tsg101 gene causes a substantial decline in the steady-state levels of the EGFR and ERBB2 [38]. This may have been the result of the dissociation of early endosomes from recycling endosomes (Figure 2). This finding is in line with an earlier report showing that TSG101 can associate with RAB11-family interacting proteins FIP3 and FIP4 [56], and this interaction might be crucial for the recycling of receptor tyrosine kinases (RTKs), including IGF-1R as discussed earlier [49]. It remains to be determined whether the reduced expression of RTKs and cellular stress responses are initiating factors or secondary events that cause TSG101 knockout cells to die.
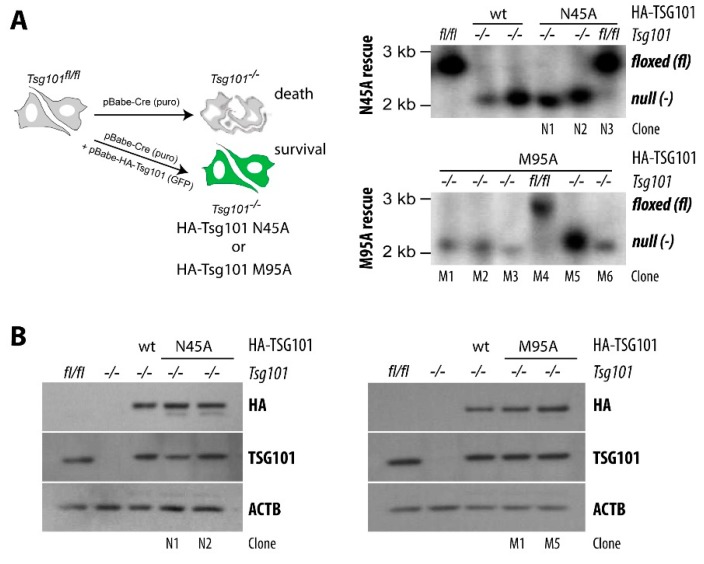
An important question is whether the proposed significant roles of TSG101 in the endocytic trafficking of ubiquitinated cargo proteins, which include RTKs, play any role in the cell survival functions of TSG101. As reviewed earlier, key functions of TSG101 in endocytic trafficking are mediated by the UEV domain of TSG101, which is able to independently bind ubiquitin and P(S/T)AP motif-containing proteins at different sites. Pornillos and coworkers [20] demonstrated that distinct point mutants of TSG101 significantly impair PTAP (M95A) or ubiquitin (N45A) binding. Given the suggested significance of these amino acids for the endosomal trafficking of viral proteins, we were surprised to find that, similar to our published knockout-rescue experiment with wildtype TSG101 [7,38], the re-expression of hemagglutinin (HA) epitope-tagged TSG101 M95A and N45A mutants was able to restore the proliferation and survival of TSG101 conditional knockout cells (Figure 3A, left panel). The lack of both endogenous Tsg101 alleles in surviving knockout rescue clones was confirmed by Southern blot (Figure 3A, right panel). Importantly, the levels of both mutant proteins were similar to endogenous TSG101 in the parental controls (Tsg101fl/fl) as well as knockout rescue clones expressing HA-tagged exogenous, wildtype (wt) TSG101 (Figure 3B). Given that TSG101 levels in normal cells are being maintained within a narrow physiological range though post-translational mechanisms, we conclude that both mutants are functionally equivalent to the wildtype protein in those cellular processes where TSG101 is crucial for cell survival. The results from the knockout rescue experiments with TSG101 mutants suggest that critical functions of TSG101 in growth and survival do not seem to be strictly dependent on an effective binding of ubiquitin or PTAP-containing proteins to its N-terminal UEV domain. Therefore, it might be possible to target regions within the UEV domain of TSG101 that are crucial for retroviral assembly and release without causing deleterious side effects that are related to other, biologically more significant functions of TSG101.
Figure 3.
Exogenous wildtype TSG101 and mutants with impaired ubiquitin (N45A) or PTAP (M95A) binding restore growth and survival of mouse fibroblasts that lack both endogenous Tsg101 alleles. (A) Experimental design of genetic rescue experiments in mouse TSG101 conditional knockout cells (left panel) to generate clones that exclusively express HA-tagged, exogenous wildtype or N45A and M95A mutants of TSG101. The right panel shows Southern blot results to verify the complete deletion of both endogenous Tsg101 alleles in individual rescue clones. (B) Western blot analyses to determine the expression of exogenous, HA-tagged TSG101 (wt, wildtype; N45A and M95A mutants) in selected rescue clones in comparison to the parental cells expressing endogenous TSG101 (Tsg101fl/fl). Detailed methods for the genetic rescue experiment, Southern blot strategy, and immunoblot reagents and methodologies can be found in our earlier publications [15,38,57].
7. Lack of Clear Evidence for a Tumor-Suppressive Role of TSG101 in Mammalian in vivo Models
Despite a wealth of information about multifaceted intracellular functions of TSG101, it is still a conundrum why a knockout of the mammalian Tsg101 gene causes cell cycle arrest and cell death instead of accelerated growth and neoplastic transformation, which would support its proposed function as a tumor suppressor. This is also the case for other vertebrate animal models such as zebrafish [53]. The essential role of TSG101 for cell survival seems to apply to all mammalian cell types that have been examined thus far including those that lack the bona fide tumor suppressor genes p53 and Cdkn2a. TSG101 is also essential for the growth of neoplastic cells that are capable of forming tumors in mice [7,15,39]. Neither haploinsufficiency nor a complete deletion of both Tsg101 alleles in somatic tissues of genetically engineered mice, such as the mammary epithelium, led to the development of benign neoplasms or cancer [14,57]. Moreover, we did not observe any mammary tumors in females where Tsg101 was deleted in the mammary epithelium that lacked both copies of the tumor suppressor p53 [39]. Instead of promoting mammary tumorigenesis, complete deficiency in TSG101 prevented the onset of mammary neoplasia in mice that overexpressed the ERBB2 oncogene [58]. This was likely a consequence of the growth inhibition of an epithelial subtype in the mammary gland that is susceptible to ERBB2-induced transformation. Deletion of both alleles of Tsg101 in established, ERBB2-transformed mammary cancer cells causes cell death (Triplett and Wagner, unpublished) [59]. These findings are similar to studies that showed that an effective downregulation of the TSG101 protein by RNA interference caused reduced proliferation and colony formation, as well as an impaired migration of human prostate (PC3), breast (MDA-MB-231, MCF- 7), and renal cancer cells (A-498 and 786-O) [60,61,62]. In SKOV-3 ovarian cancer cells, the siRNA-mediated knockdown of TSG101 led to a cell cycle arrest at the G2/M phase prior to apoptosis [63]. Silencing the expression of TSG101 in Huh7 human hepatocellular carcinoma cells resulted in abnormal actin filaments, growth arrest, and induction of autophagic cell death [64], which, as reviewed earlier, are similar phenotypic abnormalities that we observed in TSG101 conditional knockout fibroblasts [38]. On the mechanistic level, the reduced migratory features of cancer cells with a knockdown of TSG101 might be a consequence of impaired trafficking of c-SRC to focal adhesions, as well as a loss of Focal Adhesion Kinase, MAPK, and STAT3 activation [61,65].
In support of a tumor-suppressive role of TSG101, Moberg and coworkers [66] reported that lack of the TSG101 ortholog Erupted in Drosophila causes excessive cell growth, albeit in a non- cell- autonomous manner through the expression of the Notch target gene Unpaired, which is the secreted ligand that stimulates JAK/STAT signaling in the fly. It is obvious that a mechanism of a non-cell-autonomous role of TSG101 in growth suppression in the fly does not correspond to the phenotypes of a knockdown of TSG101 in normal and neoplastic cells of human and mouse origin. Although the proposed function of Erupted as a paracrine growth suppressor seems interesting, it needs to be explained why the phenotype of the Erupted-deficient fly is very different from a knockout of dVps28, which results in early embryonic lethality [67]. TSG101 and VPS28 bind directly to each other in mammalian cells [68], and more importantly, the depletion of TSG101 in human cell lines lead to a significant downregulation of the VPS28 protein [35,69]. The divergent phenotypes of Erupted and dVps28 in mutant flies might suggest that the mechanism that controls the post- translational cross-regulation of the levels of TSG101 and VPS28 in mammalian cells may not exist in Drosophila. Regardless of the nature of these discrepancies, we and others have never observed any palpable tumors in TSG101 conditional knockout mice where one or both copies of Tsg101 were deleted in a mosaic fashion in selected cell types with Cre recombinase. The same was true for a morpholino-mediated knockdown of TSG101 in zebrafish [53]. Hence, there is no conclusive experimental evidence to date using vertebrate models to suggest that TSG101 functions as a paracrine growth suppressor.
In conclusion, the various genetically engineered animal models, as well as observations from human cancer cell lines where TSG101 was knocked down with siRNAs, do not support a tumor- suppressive role of its encoded protein, either in a cell-autonomous manner or by means of juxtracrine or paracrine signaling. As stated in the opening paragraph of this review, initial reports about a loss of the TSG101 gene in human cancers could not be confirmed. Moreover, alternative splice variants of TSG101, which have been subject to intense investigations in the late 1990s, can only be detected by nested RT-PCR, and they are also present in normal tissues [9]. Most of the splice variants do not encode any functional protein and are not expressed at levels that are comparable to that of the full-length mRNA. Using Northern blot, only one strong band corresponding to the full- length message has been detected in normal tissues and mammary cancers [2,6,8]. As mentioned earlier, the lack of a significant reduction of the TSG101 protein in the original ‘TSG101-deficient’ SL6 cells should have raised questions about its assigned tumor-suppressive role as well as the validity of subsequent findings when these cells were used for mechanistic studies on the molecular functions of TSG101 without proper validation of the TSG101 protein expression using quantitative methods. To our knowledge, the transforming ability of a reduced expression of TSG101 by means of an antisense construct has never been repeated in normal human cells. Unless it can be demonstrated using genetically defined models that lowering the steady-state expression of TSG101 can trigger the onset or progression of cancer, the molecular mechanisms that were published in support of the elusive tumor-suppressive function of TSG101 should be viewed with caution.
8. TSG101 Might Function as an Oncoprotein
Investigations from various research teams including our own have shown that TSG101 is overexpressed rather than lost in a significant subset of malignancies, including breast, lung, thyroid, ovarian, and colon cancers [48,63,70,71,72,73]. According to publicly available information in the Human Protein Atlas (www.proteinatlas.org), most human cancers exhibit a moderate cytoplasmic and membranous immunoreactivity against TSG101, and the intensity of the staining might serve as a prognostic marker for selected cancer types such as hepatocellular and renal tumors. Using immunohistochemistry staining on a limited set of primary human breast cancers and normal breast tissues, our team observed that TSG101 is elevated in 50% of invasive breast cancers [48]. However, a more detailed analysis of the levels of TSG101, in particular breast cancer subtypes, using quantitative methodologies is still warranted. To illustrate this fact, we conducted a gene expression analysis on more than 3900 breast cancer cases using KM plotter (kmplot.com). Similar to the information from the Human Protein Atlas, a high or low mRNA expression of TSG101 across all cases may not serve as a prognostic marker (Logrank p = 0.49, N = 3951). In contrast, higher levels of TSG101 transcripts correlate significantly with reduced survival in luminal type A (Logrank p = 0.017, N = 2504) and in luminal type B (Logrank p = 0.0084, N = 1425) breast cancers (Ferraiuolo et al., manuscript in preparation). This implies that any studies that are designed to examine the biological significance of TSG101 and associated molecular mechanisms for a given tumor type (e.g., breast cancer) should be conducted on cell lines and animal models that resemble the main characteristics of a particular cancer-intrinsic subtype (e.g., luminal-type breast cancer models).
Similar to breast cancer, it has been reported that TSG101 is upregulated in human ovarian epithelial cells that express oncogenic HRAS or KRAS as well as in 70% of human ovarian carcinomas analyzed on tissue arrays [63,72,74]. While the normal human ovarian epithelium does not exhibit any significant expression, both low- and high-grade tumors were found to have elevated levels of TSG101. More importantly, the 5-year survival rate of patients is significantly lower (33%) when their cancer tissues exhibited higher levels of TSG101 compared to patients with a low expression of this protein (53%). These findings suggest that TSG101 might be a prognostic marker for poor overall survival in ovarian cancer [72].
The elevated expression of TSG101 in cancer compared to normal tissues may support the notion that TSG101 plays a role at particular stages of cancer initiation or progression. Experimental evidence that TSG101 overexpression might contribute to neoplastic transformation was provided in the inaugural paper by Li and Cohen [1]. This finding was validated in an independent study by Liu and coworkers [71], who isolated TSG101 as a transforming oncogene when an expression library of a highly metastatic lung adenocarcinoma cell line was introduced into mouse NIH3T3 cells. To our knowledge, the transforming ability of TSG101 overexpression has not been assessed in any other cell lines, in particular, those of human origin. As mentioned earlier, TSG101 protein levels are being maintained within a relatively narrow range of a given cell type, which may complicate the generation of suitable overexpression models. A minor or moderate expression of exogenous TSG101 results in a compensatory downregulation of the endogenous protein on the post-translational level [25]. Possible mechanisms for this phenomenon will be discussed in the next section. In contrast, a sustained overexpression of TSG101 in primary and untransformed immortalized cells, and even in cancer cell lines, often results in cell death [27]. It is a conundrum why only a few cell types are able to tolerate significantly elevated levels of exogenous TSG101. For example, sustained overexpression of epitope-tagged TSG101 under control of the Whey Acidic Protein (Wap) promoter can be achieved in functionally differentiated epithelial cells of the lactating mammary gland [48]. TSG101 overexpressing epithelial cells did not undergo apoptosis despite the activation of the STAT3, which is normally associated with the induction of apoptosis. This may have been a consequence of active STAT5 downstream of prolactin signaling, which has been demonstrated to function as a potent survival factor that can effectively overwrite STAT3-mediated pro-apoptotic signals [75]. In another transgenic model, Essandoh and colleagues [49] achieved a high expression of TSG101 in cardiomyocytes under the regulation of the α-myosin heavy chain promoter (α-MHCp). Interestingly, excess levels of TSG101 in this organ lead to enhanced cardiac function and hypertrophy of the heart due to an increase in the size of individual cardiomyocytes rather than an increase in their number or in fibrosis. Malignant transformation was not reported, and this is not surprising since cancer of the heart is extremely rare and is known to originate from connective tissue.
In contrast to the heart-specific overexpression model, a subset of transgenic females (approximately 20%) that express exogenous TSG101 under control of the Wap gene promoter developed mammary tumors after a relatively long latency [48]. At the experimental endpoint of nearly two years, more than half of all surviving WAP-TSG101 transgenic females exhibited preneoplastic or inflammatory lesions in the mammary gland. Atypical hyperplasia and squamous metaplasia were far more common in the transgenics compared to age-matched wildtype controls. The long latencies in tumor formation in this model led us to conclude that TSG101 possesses weak oncogenic properties associated with cancer initiation. Nonetheless, it is currently unknown whether all epithelial subtypes in the mammary gland are equally susceptible to TSG101-associated neoplastic transformation. While the Wap gene promotor is suitable to drive a tissue-specific expression of genes almost exclusively to the mammary epithelium, a high activation occurs predominantly in functionally differentiated alveolar cells during pregnancy and lactation, and the majority of these cells die during post-lactational mammary gland remodeling following the weaning of the offspring. As such, exogenous TSG101 is not persistently overexpressed in most epithelial cells throughout the lifetime of a female mouse. It is therefore not known whether a persistently high expression of TSG101 in diverse epithelial subtypes of the mammary gland may cause an earlier onset of mammary cancer. To our knowledge, the WAP-TSG101 transgenic mouse strain is currently the only published in vivo model that demonstrated that TSG101 can function as a transforming oncogene that causes a sporadic occurrence of preneoplastic lesions and mammary cancer. Given the long latencies of tumor formation, it is evident that secondary genetic or epigenetic changes must occur that drive the progression of bona fide cancers in this model.
9. Post-Translational Control of TSG101 Expression and Its Potential Role in Cancer
As reviewed earlier, TSG101 possesses characteristics of a housekeeping gene, and significant variations in the expression of its encoded protein between diverse cell types are, in part, a consequence of post-translational mechanisms. Given the stringent control of the TSG101 protein levels in a particular cell type, it is likely that the deregulated expression of TSG101 before or during neoplastic progression is primarily caused by an interference with its post-translational control mechanisms. The precise molecular processes that regulate the amount of TSG101 within cells are less well defined and have not been associated with any biologically relevant functions of TSG101 as an oncoprotein.
MDM2, LRSAM1/TAL, and MGRN1 are three E3 ubiquitin ligases that have been reported to control TSG101 levels in mammalian cells. Li and coworkers [54] have shown that when overexpressed in Saos-2 cells, TSG101 can interact with MDM2 and prolong the half-life of the ubiquitin ligase. In contrast, the authors also reported that MDM2 overexpression led to an accelerated decay of the TSG101 protein. Thus far, it has not been demonstrated that TSG101 levels are dependent on the functionality of MDM2 in cancers that are driven by this bona fide oncogene. If proven correct using in vivo cancer models or human specimens, the proposed mechanism would imply that TSG101 levels should be generally lower in cancers that overexpress MDM2 or lack p19Arf. Evidently, TSG101 may not function as an oncoprotein under these physiological conditions. In contrast, TSG101 may only exert oncogenic activities in the MDM2/p53 circuit when it is upregulated and able to stabilize MDM2 without being ubiquitinated and degraded by this ubiquitin ligase.
Using a yeast two-hybrid system, Amit et al. [76] identified TSG101-Associated Ligase (TAL) as a novel E3 ubiquitin ligase that ubiquitylates TSG101. TAL is also known as Leucine-rich repeat and sterile alpha motif-containing protein 1 (LRSAM1), and mutations in this gene are linked to Charcot– Marie–Tooth disease, which is a heterogeneous group of inherited motor and sensory neuropathies [77,78,79]. The N-terminal region of TAL, which contains a tandem PT/SAP motif, has been demonstrated to interact with TSG101 by binding to its UEV domain. Additionally, the central region of TAL associates with the C-terminal SB domain of TSG101 [76]. TAL is suggested to monoubiquitinate TSG101 at multiple lysine residues in the SB domain, which re-localizes the protein from being membrane-bound to the cytoplasm, thereby inactivating its sorting activities for receptor tyrosine kinases and viruses. In a subsequent study, McDonald and Martin-Serrano [80] reported that the TAL-mediated polyubiquitination of lysine residues in the C-terminus of uncomplexed TSG101 causes its proteasomal degradation. On a mechanistic level, the authors reported that the M95A point mutation in TSG101 abolished its binding to TAL. If this had been correct, we should have seen greatly elevated levels of epitope-tagged TSG101 in our genetically defined TSG101 knockout rescue clones that only express the M95A mutant protein compared to wildtype rescue clones (Figure 3). This, however, was not the case in any of the individual M95A rescue clones, supporting the previously stated notion that the interaction between TAL and TSG101 is more complex [76]. It is also likely that there are other intracellular mechanisms at play that control the level of TSG101 in the absence of TAL. Whether loss of TAL expression and functionality contributes to an upregulation of TSG101 in cancer is entirely unclear, and it has not been reported that LRSAM1 mutant mice, which exhibit only a very mild neuropathy phenotype with age [81], develop any tumors. According to the Human Protein Atlas, higher levels of TAL/LRSAM1 may serve as favorable prognostic markers in pancreatic and renal cancers, but it remains to be determined whether variations in the expression of TAL/LRSAM1 in selected cancer subtypes also correlate with changes in TSG101 protein levels.
Like the PTAP–PSAP double motif in TAL, the Mahogunin Ring Finger-1 (MGRN1) E3 ubiquitin ligase possesses a single, evolutionarily conserved PSAP amino acid sequence that can bind to the UEV domain of TSG101 [82]. MGRN1 monoubiquitinates TSG101 on multiple sites, and its siRNA- mediated depletion has been reported to impair the trafficking of EGFR to the lysosome and prolong signaling. In contrast, MGRN1 may not be required for viral budding [82,83]. MGRN1 knockout mice progressively develop spongiform neurodegeneration over the course of a year. While MGRN1 may not have a significant role in controlling the level of the TSG101 protein, this ubiquitin ligase might modify the trafficking and functionality of TSG101. By three months of age, brain tissues of MGRN1 knockout mice exhibited a build-up of insoluble, multi-ubiquitinated TSG101 that may not be correctly degraded in the proteasome and may interfere with its normal functions. If this model is correct, it could be possible that MGRN1 might mediate the oncogenic functions of TSG101. In support of this notion, the MGRN1 locus has been reported to be amplified in osteosarcomas [84] and, according to information provided in the Human Protein Atlas, most cancer types, except gliomas, showed a moderate to strong cytoplasmic staining of the MGRN1 protein. Similar to TAL, it is currently unknown whether a deregulated expression of MGRN1 significantly contributes to the expression and oncogenic properties of TSG101 in cancer.
10. Conclusions
Although TSG101 is suggested to function in a variety of cellular processes, the vast majority of published reports over the past 20 years have highlighted specific molecular mechanisms by which TSG101 may control the intracellular trafficking of cargo proteins such as RTKs and viral particles. Key observations from these studies are often highlighted as proof of the molecular underpinnings by which TSG101 may function as a growth suppressor. Then again, the validity of the proposed mechanisms is rarely, if at all, tested in genetically defined disease models or in primary human cancer tissues. At present, there is no conclusive evidence from experimental models or human tissues in support of the notion that deficiency in TSG101 or a functional loss of the protein could contribute to the genesis and progression of cancer. It has been established that TSG101 may function as a protooncogene in certain cell types. In the context of a whole organism, the biological significance of the proposed multifaceted roles of TSG101 in developmental processes and normal tissue homeostasis is still unclear. None of the proposed molecular roles sufficiently explains why cells that completely lack TSG101 enter a cell cycle arrest and die. Many of the critical functions in the intracellular trafficking of cargos as well as the binding of factors that control the post-translational modification and functionality of the TSG101 protein have been attributed to two structural features, i.e., PTAP amino acid motif-binding and ubiquitin-binding domains. Future studies using mouse models with CRISPR/Cas9-mediated targeted knock-in mutations might show whether these two domains or additional amino acid residues of TSG101 have a biologically significant role in normal development and disease.
Author Contributions
K.-U.W. and R.-M.F. wrote the manuscript, and A.A.T. assisted in analyses of TSG101 knockout mice and editing of the manuscript. K.C.M. and M.J.S. conducted the immunostaining as well as genetic knockout rescue experiments with wildtype and mutant TSG101. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Public Health Service grants CA93797 and CA219332 from the National Cancer Institute to K.-U.W. The funders had no role in the decision to publish or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Li L., Cohen S.N. Tsg101: A novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell. 1996;85:319–329. doi: 10.1016/S0092-8674(00)81111-3. [DOI] [PubMed] [Google Scholar]
- 2.Li L., Li X., Francke U., Cohen S.N. The TSG101 tumor susceptibility gene is located in chromosome 11 band p15 and is mutated in human breast cancer. Cell. 1997;88:143–154. doi: 10.1016/S0092-8674(00)81866-8. [DOI] [PubMed] [Google Scholar]
- 3.Steiner P., Barnes D.M., Harris W.H., Weinberg R.A. Absence of rearrangements in the tumour susceptibility gene TSG101 in human breast cancer. Nat. Genet. 1997;16:332–333. doi: 10.1038/ng0897-332. [DOI] [PubMed] [Google Scholar]
- 4.Li L., Francke U., Cohen S.N. Retraction. The TSG101 tumor susceptibility gene is located in chromosome 11 band p15 and is mutated in human breast cancer. Cell. 1998;93:660. doi: 10.1016/S0092-8674(00)89342-3. [DOI] [PubMed] [Google Scholar]
- 5.Zhong Q., Chen C.F., Chen Y., Chen P.L., Lee W.H. Identification of cellular TSG101 protein in multiple human breast cancer cell lines. Cancer Res. 1997;57:4225–4228. [PubMed] [Google Scholar]
- 6.Wang Q., Driouch K., Courtois S., Champeme M.H., Bieche I., Treilleux I., Briffod M., Rimokh R., Magaud J.P., Curmi P., et al. Low frequency of TSG101/CC2 gene alterations in invasive human breast cancers. Oncogene. 1998;16:677–679. doi: 10.1038/sj.onc.1201563. [DOI] [PubMed] [Google Scholar]
- 7.Krempler A., Henry M.D., Triplett A.A., Wagner K.U. Targeted deletion of the Tsg101 gene results in cell cycle arrest at G1/S and p53-independent cell death. J. Biol. Chem. 2002;277:43216–43223. doi: 10.1074/jbc.M207662200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner K.U., Dierisseau P., Rucker E.B., Robinson G.W., Hennighausen L. Genomic architecture and transcriptional activation of the mouse and human tumor susceptibility gene TSG101: Common types of shorter transcripts are true alternative splice variants. Oncogene. 1998;17:2761–2770. doi: 10.1038/sj.onc.1202529. [DOI] [PubMed] [Google Scholar]
- 9.Gayther S.A., Barski P., Batley S.J., Li L., de Foy K.A., Cohen S.N., Ponder B.A., Caldas C. Aberrant splicing of the TSG101 and FHIT genes occurs frequently in multiple malignancies and in normal tissues and mimics alterations previously described in tumours. Oncogene. 1997;15:2119–2126. doi: 10.1038/sj.onc.1201591. [DOI] [PubMed] [Google Scholar]
- 10.Lee M.P., Feinberg A.P. Aberrant splicing but not mutations of TSG101 in human breast cancer. Cancer Res. 1997;57:3131–3134. [PubMed] [Google Scholar]
- 11.Sun Z., Pan J., Hope W.X., Cohen S.N., Balk S.P. Tumor susceptibility gene 101 protein represses androgen receptor transactivation and interacts with p300. Cancer. 1999;86:689–696. doi: 10.1002/(SICI)1097-0142(19990815)86:4<689::AID-CNCR19>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Kozak M. Recognition of AUG and alternative initiator codons is augmented by G in position +4 but is not generally affected by the nucleotides in positions +5 and +6. EMBO J. 1997;16:2482–2492. doi: 10.1093/emboj/16.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner K.U., Dierisseau P., Hennighausen L. Assignment of the murine tumor susceptibility gene 101 (tsg101) and a processed tsg101 pseudogene (tsg101-ps1) to mouse chromosome 7 band B5 and chromosome 15 band D1 by in situ hybridization. Cytogenet. Cell Genet. 1999;84:87–88. doi: 10.1159/000015221. [DOI] [PubMed] [Google Scholar]
- 14.Ruland J., Sirard C., Elia A., MacPherson D., Wakeham A., Li L., Luis D.L.P., Cohen S.N., Mak T.W. p53 Accumulation, defective cell proliferation, and early embryonic lethality in mice lacking tsg101. Proc. Natl. Acad. Sci. USA. 2001;98:1859–1864. doi: 10.1073/pnas.98.4.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner K.U., Krempler A., Qi Y., Park K., Henry M.D., Triplett A.A., Riedlinger G., Rucker III E.B., Hennighausen L. Tsg101 Is Essential for Cell Growth, Proliferation, and Cell Survival of Embryonic and Adult Tissues. Mol. Cell Biol. 2003;23:150–162. doi: 10.1128/MCB.23.1.150-162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koonin E.V., Abagyan R.A. TSG101 may be the prototype of a class of dominant negative ubiquitin regulators. Nat. Genet. 1997;16:330–331. doi: 10.1038/ng0897-330. [DOI] [PubMed] [Google Scholar]
- 17.Ponting C.P., Cai Y.D., Bork P. The breast cancer gene product TSG101: A regulator of ubiquitination? J. Mol. Med. 1997;75:467–469. [PubMed] [Google Scholar]
- 18.Garrus J.E., von Schwedler U.K., Pornillos O.W., Morham S.G., Zavitz K.H., Wang H.E., Wettstein D.A., Stray K.M., Cote M., Rich R.L., et al. Tsg101 and the vacuolar protein sorting pathway are essential for hiv-1 budding. Cell. 2001;107:55–65. doi: 10.1016/S0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 19.Katzmann D.J., Babst M., Emr S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/S0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 20.Pornillos O., Alam S.L., Rich R.L., Myszka D.G., Davis D.R., Sundquist W.I. Structure and functional interactions of the Tsg101 UEV domain. EMBO J. 2002;21:2397–2406. doi: 10.1093/emboj/21.10.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pornillos O., Alam S.L., Davis D.R., Sundquist W.I. Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein. Nat. Struct. Biol. 2002;9:812–817. doi: 10.1038/nsb856. [DOI] [PubMed] [Google Scholar]
- 22.Pornillos O., Higginson D.S., Stray K.M., Fisher R.D., Garrus J.E., Payne M., He G.P., Wang H.E., Morham S.G., Sundquist W.I. HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein. J. Cell Biol. 2003;162:425–434. doi: 10.1083/jcb.200302138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundquist W.I., Schubert H.L., Kelly B.N., Hill G.C., Holton J.M., Hill C.P. Ubiquitin recognition by the human TSG101 protein. Mol. Cell. 2004;13:783–789. doi: 10.1016/S1097-2765(04)00129-7. [DOI] [PubMed] [Google Scholar]
- 24.Maucuer A., Camonis J.H., Sobel A. Stathmin interaction with a putative kinase and coiled-coil-forming protein domains. Proc. Natl. Acad. Sci. USA. 1995;92:3100–3104. doi: 10.1073/pnas.92.8.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng G.H., Lih C.J., Cohen S.N. TSG101 protein steady-state level is regulated post-translationally by an evolutionarily conserved COOH-terminal sequence. Cancer Res. 2000;60:1736–1741. [PubMed] [Google Scholar]
- 26.Lu Q., Hope L.W., Brasch M., Reinhard C., Cohen S.N. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl. Acad. Sci. USA. 2003;100:7626–7631. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Q., Chen Y., Jones D., Lee W.H. Perturbation of TSG101 protein affects cell cycle progression. Cancer Res. 1998;58:2699–2702. [PubMed] [Google Scholar]
- 28.Xie W., Li L., Cohen S.N. Cell cycle-dependent subcellular localization of the TSG101 protein and mitotic and nuclear abnormalities associated with TSG101 deficiency. Proc. Natl. Acad. Sci. USA. 1998;95:1595–1600. doi: 10.1073/pnas.95.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hittelman A.B., Burakov D., Iniguez-Lluhi J.A., Freedman L.P., Garabedian M.J. Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins. EMBO J. 1999;18:5380–5388. doi: 10.1093/emboj/18.19.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y.S., Chen Y.J., Cohen S.N., Cheng T.H. Identification of TSG101 functional domains and p21 loci required for TSG101-mediated p21 gene regulation. PloS ONE. 2013;8:e79674. doi: 10.1371/journal.pone.0079674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh H., Mammucari C., Nenci A., Cabodi S., Cohen S.N., Dotto G.P. Negative regulation of cell growth and differentiation by TSG101 through association with p21Cip1/WAF1. Proc. Natl. Acad. Sci. USA. 2002;99:5430–5435. doi: 10.1073/pnas.082123999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H.H., Elia N., Ghirlando R., Lippincott-Schwartz J., Hurley J.H. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science. 2008;322:576–580. doi: 10.1126/science.1162042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Kane T., Tipper C., Spatrick P., Jenness D.D. Yeast mutants affecting possible quality control of plasma membrane proteins. Mol. Cell Biol. 1999;19:3588–3599. doi: 10.1128/MCB.19.5.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babst M., Odorizzi G., Estepa E.J., Emr S.D. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- 35.Doyotte A., Russell M.R., Hopkins C.R., Woodman P.G. Depletion of TSG101 forms a mammalian “Class E” compartment: a multicisternal early endosome with multiple sorting defects. J. Cell Sci. 2005;118:3003–3017. doi: 10.1242/jcs.02421. [DOI] [PubMed] [Google Scholar]
- 36.Bishop N., Horman A., Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J. Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raiborg C., Malerod L., Pedersen N.M., Stenmark H. Differential functions of Hrs and ESCRT proteins in endocytic membrane trafficking. Exp. Cell Res. 2008;314:801–813. doi: 10.1016/j.yexcr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Morris C.R., Stanton M.J., Manthey K.C., Oh K.B., Wagner K.U. A knockout of the Tsg101 gene leads to decreased expression of ErbB receptor tyrosine kinases and induction of autophagy prior to cell death. PLoS ONE. 2012;7:e34308. doi: 10.1371/journal.pone.0034308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carstens M.J., Krempler A., Triplett A.A., van Lohuizen M., Wagner K.U. Cell cycle arrest and cell death are controlled by p53-dependent and p53-independent mechanisms in Tsg101-deficient cells. J. Biol. Chem. 2004;279:35984–35994. doi: 10.1074/jbc.M400408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Schwedler U.K., Stuchell M., Muller B., Ward D.M., Chung H.Y., Morita E., Wang H.E., Davis T., He G.P., Cimbora D.M., et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/S0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 41.VerPlank L., Bouamr F., LaGrassa T.J., Agresta B., Kikonyogo A., Leis J., Carter C.A. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag) Proc. Natl. Acad. Sci. USA. 2001;98:7724–7729. doi: 10.1073/pnas.131059198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin-Serrano J., Zang T., Bieniasz P.D. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- 43.Strickland M E.L., Watanabe S., Khan M., Strub M.P., Luan C.H., Powell M.D., Leis J., Tjandra N., Carter C.A. Tsg101 chaperone function revealed by HIV-1 assembly inhibitors. Nat. Comm. 2017;8 doi: 10.1038/s41467-017-01426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thery C., Boussac M., Veron P., Ricciardi-Castagnoli P., Raposo G., Garin J., Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 45.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L.F., Thery C., Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 47.Stanton M.J., Manthey K.C., Triplett A.A., Wagner K.U. Novel Roles of Tsg101 in Endocytic Recycling Revealed by Analyses in Cre-Inducible Tsg101 Knockout Cells. University of Nebraska Medical Center; Omaha, NE, USA: 2011. unpublished. [Google Scholar]
- 48.Oh K.B., Stanton M.J., West W.W., Todd G.L., Wagner K.U. Tsg101 is upregulated in a subset of invasive human breast cancers and its targeted overexpression in transgenic mice reveals weak oncogenic properties for mammary cancer initiation. Oncogene. 2007;26:5950–5959. doi: 10.1038/sj.onc.1210401. [DOI] [PubMed] [Google Scholar]
- 49.Essandoh K., Deng S., Wang X., Jiang M., Mu X., Peng J., Li Y., Peng T., Wagner K.U., Rubinstein J., et al. Tsg101 positively regulates physiologic-like cardiac hypertrophy through FIP3-mediated endosomal recycling of IGF-1R. FASEB J. 2019;33:7451–7466. doi: 10.1096/fj.201802338RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Essandoh K., Wang X., Huang W., Deng S., Gardner G., Mu X., Li Y., Kranias E.G., Wang Y., Fan G.C. Tumor susceptibility gene 101 ameliorates endotoxin-induced cardiac dysfunction by enhancing Parkin-mediated mitophagy. J. Biol. Chem. 2019;294:18057–18068. doi: 10.1074/jbc.RA119.008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J., Wende A.R., Sena S., Theobald H.A., Soto J., Sloan C., Wayment B.E., Litwin S.E., Holzenberger M., LeRoith D., et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol. Endocrinol. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker W.P., Oehler A., Edinger A.L., Wagner K.U., Gunn T.M. Oligodendroglial deletion of ESCRT-I component TSG101 causes spongiform encephalopathy. Biol. Cell. 2016;108:324–337. doi: 10.1111/boc.201600014. [DOI] [PubMed] [Google Scholar]
- 53.Langheinrich U., Hennen E., Stott G., Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr. Biol. 2002;12:2023–2028. doi: 10.1016/S0960-9822(02)01319-2. [DOI] [PubMed] [Google Scholar]
- 54.Li L., Liao J., Ruland J., Mak T.W., Cohen S.N. A TSG101/MDM2 regulatory loop modulates MDM2 degradation and MDM2/p53 feedback control. Proc. Natl. Acad. Sci. USA. 2001;98:1619–1624. doi: 10.1073/pnas.98.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Razi M., Futter C.E. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol. Biol. Cell. 2006;17:3469–3483. doi: 10.1091/mbc.e05-11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horgan C.P., Hanscom S.R., Kelly E.E., McCaffrey M.W. Tumor Susceptibility Gene 101 (TSG101) Is a Novel Binding-Partner for the Class II Rab11-FIPs. PloS ONE. 2012;7:e32030. doi: 10.1371/journal.pone.0032030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krempler A., Qi Y., Triplett A.A., Zhu J., Rui H., Wagner K.U. Generation of a conditional knockout allele for the Janus kinase 2 (Jak2) gene in mice. Genesis. 2004;40:52–57. doi: 10.1002/gene.20063. [DOI] [PubMed] [Google Scholar]
- 58.Henry M.D., Triplett A.A., Oh K.B., Smith G.H., Wagner K.U. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–6985. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- 59.Triplett A.A., Oh K.B., Wagner K.U. Deletion of both TSG101 alleles in Erbb2-induced mammary tumors results in cell death. University of Nebraska Medical Center; Omaha, NE, USA: 2007. unpublished. [Google Scholar]
- 60.Zhu G., Gilchrist R., Borley N., Chng H.W., Morgan M., Marshall J.F., Camplejohn R.S., Muir G.H., Hart I.R. Reduction of TSG101 protein has a negative impact on tumor cell growth. Int. J. Cancer. 2004;109:541–547. doi: 10.1002/ijc.20014. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y., Song M., Cui Z.S., Li C.Y., Xue X.X., Yu M., Lu Y., Zhang S.Y., Wang E.H., Wen Y.Y. Down-regulation of TSG101 by small interfering RNA inhibits the proliferation of breast cancer cells through the MAPK/ERK signal pathway. Histol. Histopathol. 2011;26:87–94. doi: 10.14670/hh-26.87. [DOI] [PubMed] [Google Scholar]
- 62.Xu C., Zheng J. siRNA against TSG101 reduces proliferation and induces G0/G1 arrest in renal cell carcinoma - involvement of c-myc, cyclin E1, and CDK2. Cell Mol. Biol. Lett. 2019;24:7. doi: 10.1186/s11658-018-0124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young T.W., Mei F.C., Rosen D.G., Yang G., Li N., Liu J., Cheng X. Up-regulation of tumor susceptibility gene 101 protein in ovarian carcinomas revealed by proteomics analyses. Mol. Cell Proteom. 2007;6:294–304. doi: 10.1074/mcp.M600305-MCP200. [DOI] [PubMed] [Google Scholar]
- 64.Shao Z., Ji W., Liu A., Qin A., Shen L., Li G., Zhou Y., Hu X., Yu E., Jin G. TSG101 Silencing Suppresses Hepatocellular Carcinoma Cell Growth by Inducing Cell Cycle Arrest and Autophagic Cell Death. Med. Sci. Monit. 2015;21:3371–3379. doi: 10.12659/MSM.894447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tu C., Ortega-Cava C.F., Winograd P., Stanton M.J., Reddi A.L., Dodge I., Arya R., Dimri M., Clubb R.J., Naramura M., et al. Endosomal-sorting complexes required for transport (ESCRT) pathway-dependent endosomal traffic regulates the localization of active Src at focal adhesions. Proc. Natl. Acad. Sci. USA. 2010;107:16107–16112. doi: 10.1073/pnas.1009471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moberg K.H., Schelble S., Burdick S.K., Hariharan I.K. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 67.Sevrioukov E.A., Moghrabi N., Kuhn M., Kramer H. A mutation in dVps28 reveals a link between a subunit of the endosomal sorting complex required for transport-I complex and the actin cytoskeleton in Drosophila. Mol. Biol. Cell. 2005;16:2301–2312. doi: 10.1091/mbc.e04-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bishop N., Woodman P. TSG101/mammalian VPS23 and mammalian VPS28 interact directly and are recruited to VPS4-induced endosomes. J. Biol. Chem. 2001;276:11735–11742. doi: 10.1074/jbc.M009863200. [DOI] [PubMed] [Google Scholar]
- 69.Bache K.G., Slagsvold T., Cabezas A., Rosendal K.R., Raiborg C., Stenmark H. The growth-regulatory protein HCRP1/hVps37A is a subunit of mammalian ESCRT-I and mediates receptor down-regulation. Mol. Biol. Cell. 2004;15:4337–4346. doi: 10.1091/mbc.e04-03-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu R.T., Huang C.C., You H.L., Chou F.F., Hu C.C., Chao F.P., Chen C.M., Cheng J.T. Overexpression of tumor susceptibility gene TSG101 in human papillary thyroid carcinomas. Oncogene. 2002;21:4830–4837. doi: 10.1038/sj.onc.1205612. [DOI] [PubMed] [Google Scholar]
- 71.Liu F., Yu Y., Jin Y., Fu S. TSG101, identified by screening a cancer cDNA library and soft agar assay, promotes cell proliferation in human lung cancer. Mol. Biol. Rep. 2010;37:2829–2838. doi: 10.1007/s11033-009-9835-5. [DOI] [PubMed] [Google Scholar]
- 72.Young T.W., Rosen D.G., Mei F.C., Li N., Liu J., Wang X.F., Cheng X. Up-regulation of tumor susceptibility gene 101 conveys poor prognosis through suppression of p21 expression in ovarian cancer. Clin. Cancer Res. 2007;13:3848–3854. doi: 10.1158/1078-0432.CCR-07-0337. [DOI] [PubMed] [Google Scholar]
- 73.Ma X.R., Edmund Sim U.H., Pauline B., Patricia L., Rahman J. Overexpression of WNT2 and TSG101 genes in colorectal carcinoma. Trop. Biomed. 2008;25:46–57. [PubMed] [Google Scholar]
- 74.Cheng X., Young T.W., Mei F.C. Proteomics analyses of ovarian cancer using genetically defined human ovarian cancer models. Front Biosci. 2007;12:5166–5174. doi: 10.2741/2556. [DOI] [PubMed] [Google Scholar]
- 75.Creamer B.A., Sakamoto K., Schmidt J.W., Triplett A.A., Moriggl R., Wagner K.U. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in Akt1. Mol. Cell Biol. 2010;30:2957–2970. doi: 10.1128/MCB.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amit I., Yakir L., Katz M., Zwang Y., Marmor M.D., Citri A., Shtiegman K., Alroy I., Tuvia S., Reiss Y., et al. Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev. 2004;18:1737–1752. doi: 10.1101/gad.294904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nicolaou P., Cianchetti C., Minaidou A., Marrosu G., Zamba-Papanicolaou E., Middleton L., Christodoulou K. A novel LRSAM1 mutation is associated with autosomal dominant axonal Charcot-Marie-Tooth disease. Eur. J. Hum. Genet. 2013;21:190–194. doi: 10.1038/ejhg.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weterman M.A., Sorrentino V., Kasher P.R., Jakobs M.E., van Engelen B.G., Fluiter K., de Wissel M.B., Sizarov A., Nurnberg G., Nurnberg P., et al. A frameshift mutation in LRSAM1 is responsible for a dominant hereditary polyneuropathy. Hum. Mol. Genet. 2012;21:358–370. doi: 10.1093/hmg/ddr471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guernsey D.L., Jiang H., Bedard K., Evans S.C., Ferguson M., Matsuoka M., Macgillivray C., Nightingale M., Perry S., Rideout A.L., et al. Mutation in the gene encoding ubiquitin ligase LRSAM1 in patients with Charcot-Marie-Tooth disease. Plos Genet. 2010:6. doi: 10.1371/journal.pgen.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McDonald B., Martin-Serrano J. Regulation of Tsg101 Expression by the Steadiness Box: A Role of Tsg101-associated Ligase. Mol. Biol. Cell. 2008;19:754–763. doi: 10.1091/mbc.e07-09-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bogdanik L.P., Sleigh J.N., Tian C., Samuels M.E., Bedard K., Seburn K.L., Burgess R.W. Loss of the E3 ubiquitin ligase LRSAM1 sensitizes peripheral axons to degeneration in a mouse model of Charcot-Marie-Tooth disease. Dis. Models Mech. 2013;6:780–792. doi: 10.1242/dmm.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim B.Y., Olzmann J.A., Barsh G.S., Chin L.S., Li L. Spongiform neurodegeneration-associated E3 ligase Mahogunin ubiquitylates TSG101 and regulates endosomal trafficking. Mol. Biol. Cell. 2007;18:1129–1142. doi: 10.1091/mbc.e06-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiao J., Sun K., Walker W.P., Bagher P., Cota C.D., Gunn T.M. Abnormal regulation of TSG101 in mice with spongiform neurodegeneration. Biochim. Biophys. Acta. 2009;1792:1027–1035. doi: 10.1016/j.bbadis.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Man T.K., Lu X.Y., Jaeweon K., Perlaky L., Harris C.P., Shah S., Ladanyi M., Gorlick R., Lau C.C., Rao P.H. Genome-wide array comparative genomic hybridization analysis reveals distinct amplifications in osteosarcoma. BMC Cancer. 2004;4:45. doi: 10.1186/1471-2407-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]