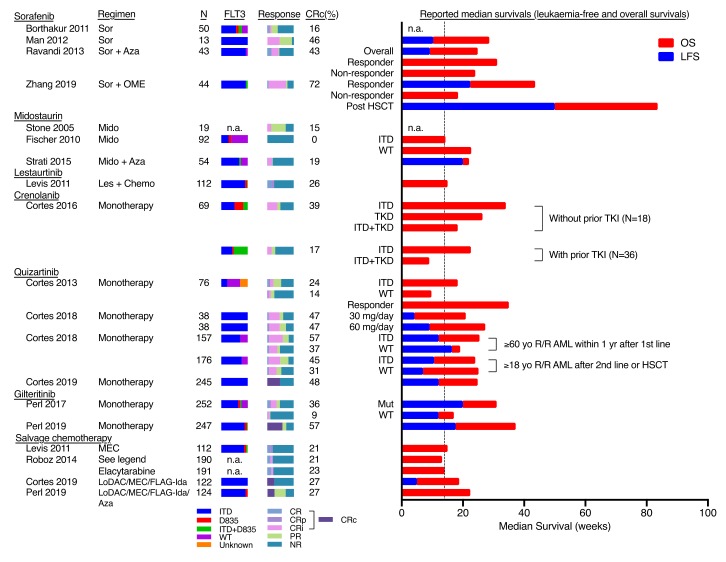
Figure 1.
Results of clinical trials involving Fms-Like Tyrosine kinase 3 (FLT3) inhibitors on relapsed/refractory AML. The reported sample size (N), percentage of FLT3-ITD, D835, ITD/D835 and wildtype (WT), and response rate and duration of reported clinical trials on the use of FLT3 inhibitors on relapsed/refractory (R/R) AML were shown here. The response duration was represented as bar charts (right) for graphical presentation and it was not intended for direct statistical comparison between studies. The vertical dotted line represented the estimated pooled median overall survival in R/R patients treated with salvage chemotherapy (around 14 weeks). CR: complete remission; CRp: complete remission with incomplete platelet count; CRi: complete remission with incomplete haematological recovery; PR: partial response; NR: no response; CRc: composite complete remission rate = CR + CRp + CRi; Sor: sorafenib; Aza: Azacytidine; OME: omacetaxine mepesuccinate; Mido: midostaurin; Les: Lestaurtinib; MEC: mitoxantrone, etoposide, cytarabine; FLAG-Ida: Fludarabine, cytarabine, G-CSF, Idarubicin; LoDAC: low-dose cytarabine; HiDAC: high-dose cytarabine; TKI: tyrosine kinase inhibitors; TKI: tyrosine kinase inhibitor(s). The chemotherapy reported in Roboz et al. (2014) included investigators’ choice among 7 salvage regimens: HiDAC, MEC, FLAG/FLAG-Ida, LoDAC, hypomethylating agents, hydroxyurea, or supportive care. Reference (top to bottom): [18,10,19,20,21,17,22,23,24,25,26,27,4,28,3,23,29,4,3].