Figure 1: Proposed mechanisms whereby adipose tissue controls systemic insulin sensitivity.
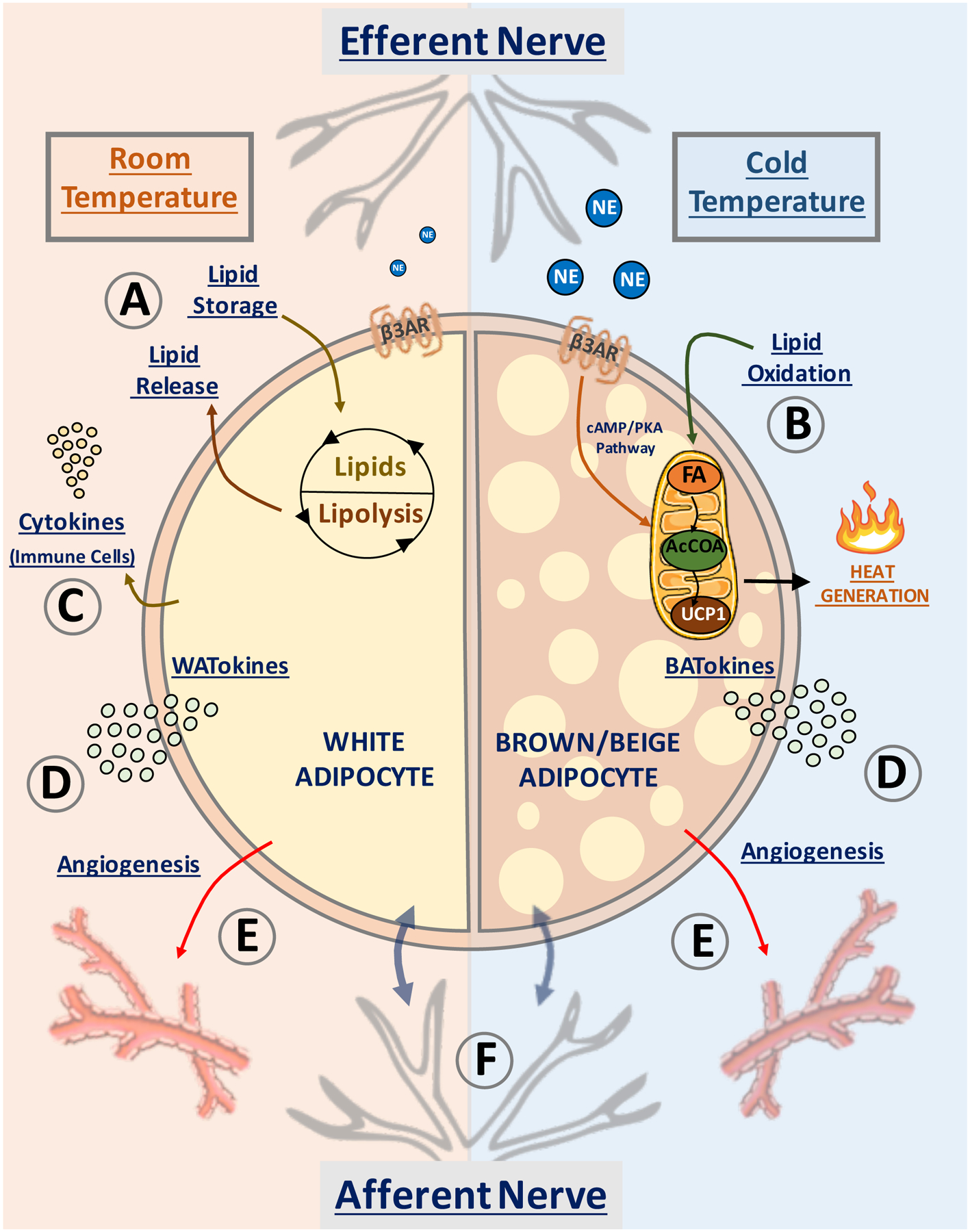
Depicted are pathways in white adipocytes (left semicircle) and beige/brown adipocytes (right semicircle) that have been proposed to affect whole body insulin sensitivity and systemic metabolism. At room temperature (22°C) and above, most of WAT is composed of white adipocytes (left), while at low temperature (6°C) brown/beige adipocytes appear in WAT (right). (A) White adipocytes promote fatty acid esterification into triglycerides for storage, sequestering fat away from liver and skeletal muscle to prevent “lipotoxicity”. (B) Sustained release of norepinephrine (NE) by adipose efferent nerves activates β3-adrenergic (β3AR) receptor and induces “beige” adipocyte formation within white adipose tissue. Beige adipocytes display increased mitochondrial density and high capacity for fatty acid (FA) oxidation into acetyl-CoA (AcCOA) which fuels heat production via mitochondrial uncoupling protein-1 (UCP1) within the electron transport chain. (C) White adipocytes upregulate resident immune cells in obesity, releasing cytokines into the circulation. (D) Secretion of white adipocyte-derived bioactive molecules (denoted WATokines) and beige or brown adipocyte-derived factors (denoted BATokines) may modulate (E) adipose vascularization and (F) activate local afferent nerve fibers. These factors can also be released into the circulation to affect distant tissues.
