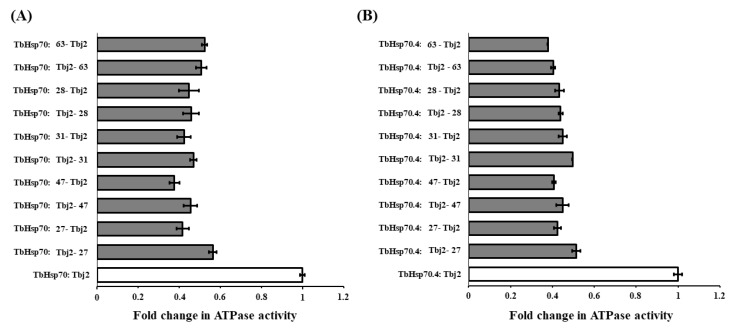
Figure 5.
The compounds do not disrupt the interaction of Tbj2 with the T. brucei Hsp70s. Investigation into whether varying the order of addition of the reaction components impacts inhibition of Tbj2-stimulated ATPase activity of the T. brucei Hsp70s, TbHsp70 (A) and TbHsp70.4 (B), and elucidation of whether the small molecules disrupt Hsp70/J-protein interaction. Bars denoted with Hsp70: small molecule-J-protein indicate reactions in which the Hsp70 was pre-incubated with the small molecule prior to addition of the J-protein and vice versa. Results are represented as fold change in the untreated J-stimulated Hsp70 ATPase activity (white bars) in relation to J-stimulated ATPase activity of the Hsp70s in the presence of small molecules at varying concentrations (grey bars). Standard deviations were obtained from two replicate assays on three independent batches of proteins. However, no significant difference was observed in the inhibition of the J-stimulated ATPase activity for both T. brucei Hsp70s by varying the order of addition of the reaction components (p < 0.05).