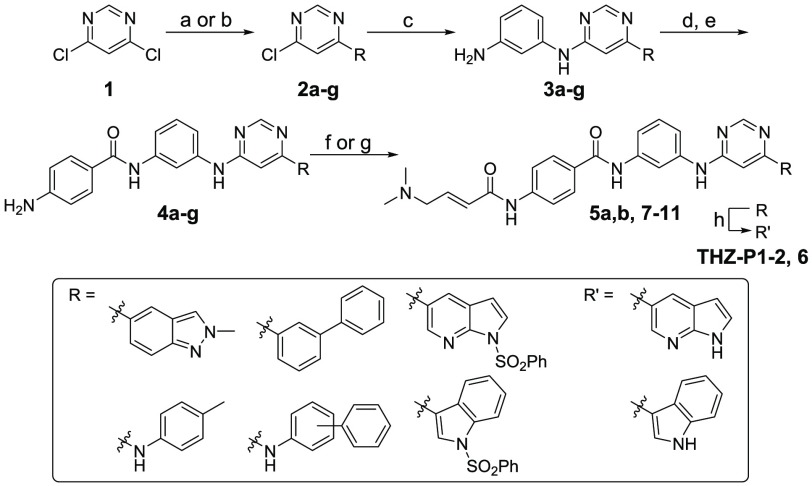
Scheme 1. Synthesis of THZ-P1-2 and Compounds 6–11.
Reagents and conditions: (a) (HO)2B-R or respective pinacol boronic acid esters, NaHCO3, Pd(PPh3)2Cl2, ACN/H2O, 90–100 °C, 2.5–19 h, 53–83% yield; (b) 4-methylaniline, TEA, BuOH, 140 °C, 1 h or 3/4-phenylaniline, TEA, EtOH, 120 °C, 3 h, 99%-quant. yield; (c) m-phenylenediamine, DIEA, NMP, 140–160 °C, 1.5 h to 2 d, 37–95% yield; (d) 4-nitrobenzoyl chloride, pyridine or TEA/DCM, rt, 5 h or 4-((tert-butoxycarbonyl)amino)benzoic acid, HATU, Hunig’s Base, DMF, rt, 1 h, then TFA/DCM, rt, 2 h, 37% yield; (e) SnCl2·2H2O, EtOAc/MeOH, 80 °C, 4.5 h to 2 d, or H2, 10% Pd/C, EtOAc/MeOH or MeOH, rt, 16 h, 4–87% yield over 2 steps; (f) 4-bromocrotonyl chloride, DIEA, DCM or ACN, 0 °C, then dimethylamine, THF, rt, 1 h, 24–71% yield; (g) 4-dimethylaminocrotonic acid, DIEA, HATU, DCM, or oxalylic chloride, DMF rt, 1–12 h, 6–30% yield; (h) 1.0 M NaOH/1,4-dioxane, rt, 4–6 h, 8–60% yield.