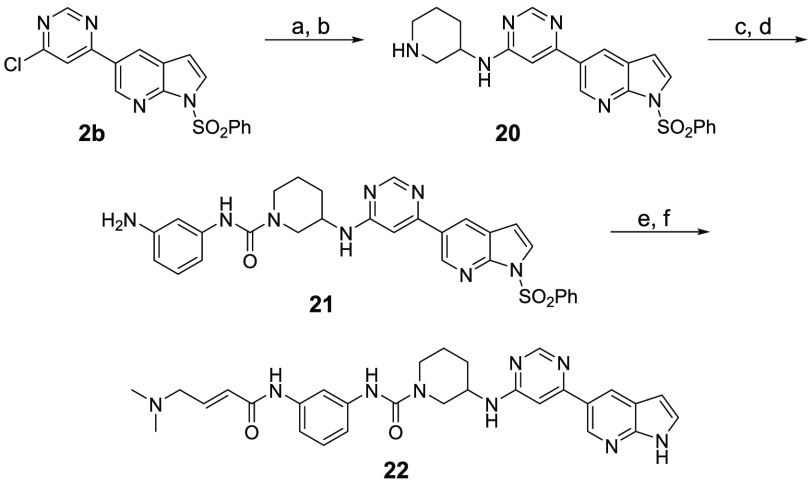
Scheme 3. Synthesis of Compound 22.
Reagents and conditions: (a) tert-butyl 3-aminopiperidine-1-carboxylate, DIEA, NMP, 110 °C, overnight, 50% yield; (b) TFA/DCM, rt, overnight, quant. yield; (c) 3-nitroaniline, triphosgene, TEA, DCM, 0 °C, 2 h, then rt, overnight, 83% yield; (d) SnCl2, EtOAc/MeOH, reflux, overnight, 74% yield; (e) 4-bromocrotonyl chloride, DIEA, DCM, 0 °C, 5 min, then dimethylamine (2.0 M in THF), rt, 1 h, 68% yield; (f) 1.0 M NaOH/1,4-dioxane, 10 °C to rt, 4 h, 30% yield.