Figure 1.
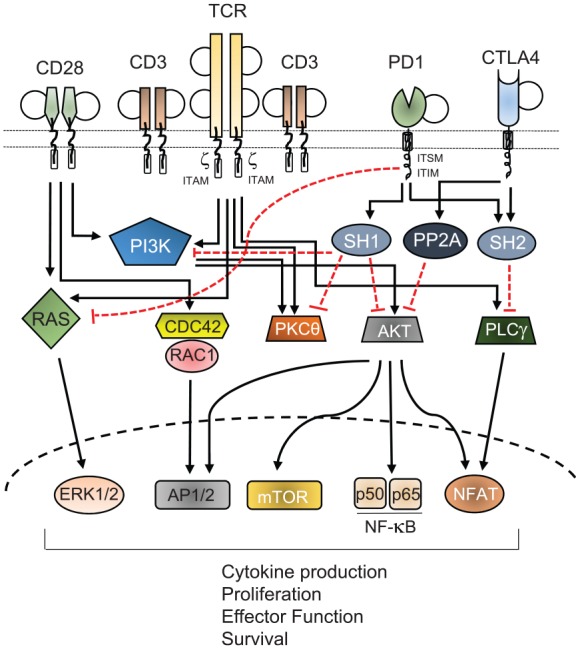
TCR/CD3 complex and CD28 downstream signaling pathways leading to activation of transcription of genes for cytokines, chemokines, cell division, activation of effector function, and survival. The coinhibitory receptors programmed cell death 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) suppress T-cell activation and function through the recruitment of the phosphatases SH2 domain-containing tyrosine phosphatase 1 (SHP1), SHP2 and serine/threonine protein phosphatase 2A (PP2A) via their ITAM, ITIM, or ITSM motif. These phosphatases dephosphorylate critical serine/threonine protein kinases PI3K, AKT, and PLCθ that play roles in multiple cellular processes for stimulation of T cells. PD-1 inhibits the RAS-extracellular signal-regulated kinase (ERK) pathway and CTLA-4 inhibits PLCγ and thereby the NFAT transcriptional activity. NFAT activation and its nuclear translocation requires cooperation of calmodulin, a well-known calcium sensor protein, which activates the serine/threonine phosphatase calcineurin. Engagement of PD-1 receptor with PD-L1 or PD-L2 recruits SHP2 phosphatase to its cytoplasmic domain, which functions to inhibit TCR signaling pathway by preventing ZAP70 phosphorylation and its association with CD3ζ at TCR complex.
ζ, TCR homodimeric domain; AKT, protein-kinase B; AP, activator protein; CDC42, cell division control protein 42 homolog; FOXO1, forkhead box protein O1; ITAM, T-cell immunoreceptor tyrosine-based activation motif; ITIM, T-cell immunoreceptor tyrosine-based inhibitory motif; ITSM, immunoreceptor tyrosine-based switch motif; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NFAT, nuclear factor of activated T cells; PKCθ, protein kinase Cθ; PLCγ, phospholipase Cγ; RAC1, Ras-related C3 botulinum toxin substrate 1; TCR, T-cell receptor.
