FIGURE 1.
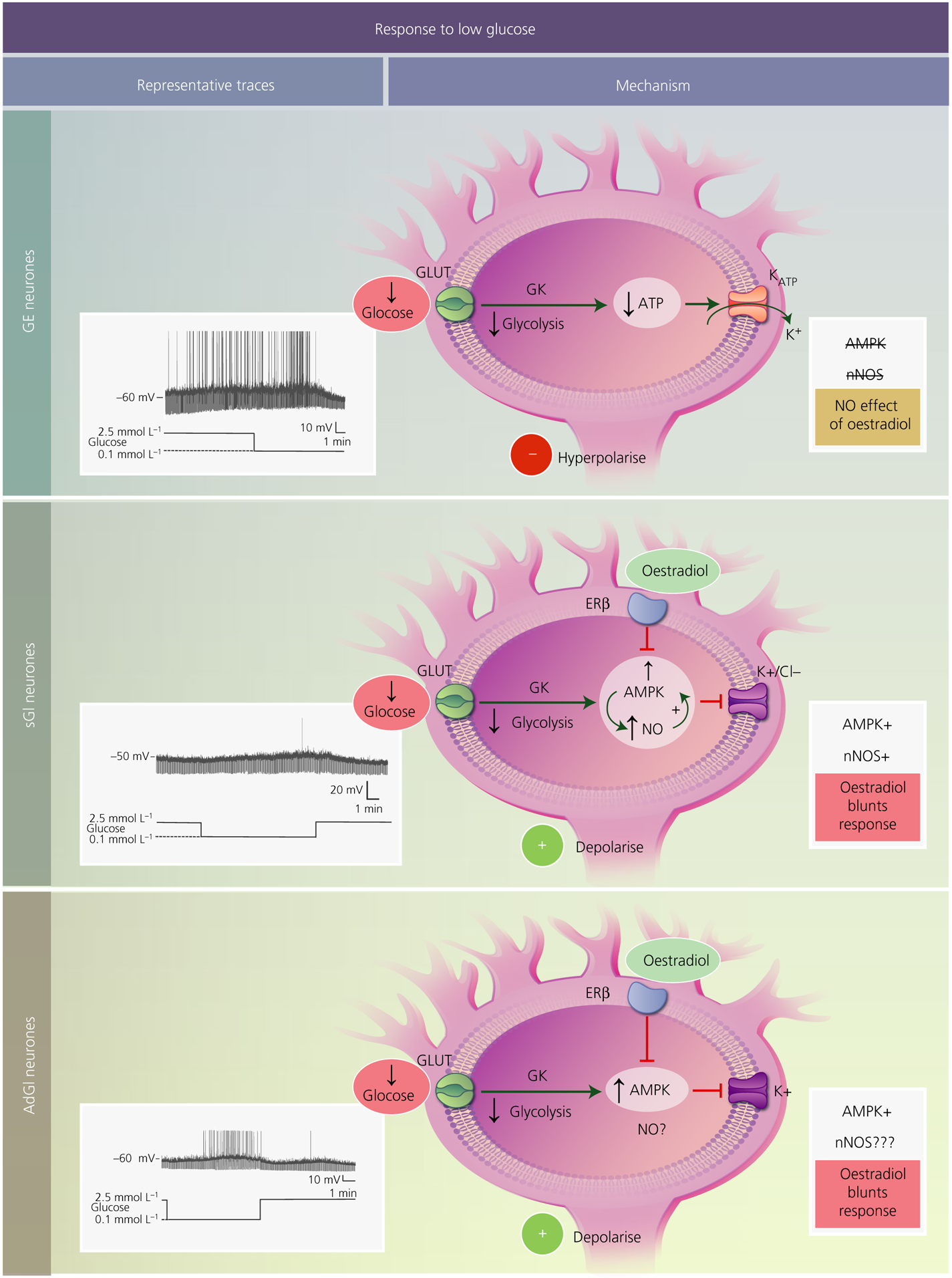
Mechanisms underlying the response of ventromedial hypothalamus (VMH) glucose-sensing neurones to low glucose. In all subtypes, glucose is transported into the cell most likely via a canonical glucose transporter (GLUT). Once inside the neurone, it is phosphorylated by the pancreatic form of glucokinase (GK) and proceeds through glycolysis. Decreased glucose leads to a reduction in ATP levels. For glucose-excited (GE) neurones (top), decreased ATP opens the ATP-sensitive potassium channel (KATP), leading to potassium efflux and hyperpolarisation. VMH GE neurones do not use AMP-activated protein kinase (AMPK) or neuronal nitric oxide synthase (nNOS) to sense glucose, nor are they regulated by oestradiol. Glucose-inhibited neurones are either constantly activated in low glucose (sustained or sGI neurones; middle) or show a transient activation that reverses when still in the presence of low glucose (adapting or AdGI neurones; lower). For sGI neurones, low glucose activates AMPK leading to phosphorylation of nNOS and increased NO production. NO, in turn, further activates AMPK, leading to closure of either potassium or chloride channels and cellular depolarisation. Oestradiol, through the oestrogen receptor (ER)β blunts activation in low glucose by inhibiting AMPK. Similar to sGI neurones, AdGI neurones require AMPK for potassium channel closure and activation in low glucose. Oestradiol similarly affects glucose sensitivity by blocking AMPK activation. It is not yet known whether nNOS is involved in glucose sensing by AdGI neurones. Representative current clamp recordings of each subtype of VMH glucose-sensing neurones in brain slices are shown to the left. The membrane potential in 2.5 mmol L−1 glucose is shown to the left of each trace. Upward deflections represent action potentials and downward deflections are the membrane voltage response to a constant hyperpolarising current pulse. Membrane resistance is directly proportional to these voltage responses. Thus, low glucose decreases membrane resistance in GE neurones, indicating ion channel opening (KATP). Conversely, low glucose increases resistance in sGI and AdGI neurones indicating closure of potassium or chloride channels. Interestingly, as demonstrated in the present study and in Santiago et al,1 AdGI neurones show a smaller transient activation when glucose levels return to 2.5 mmol L−1, suggesting that they are sensitive not only to glucose concentration per se, but also to the actual change in glucose level
