FIGURE 2.
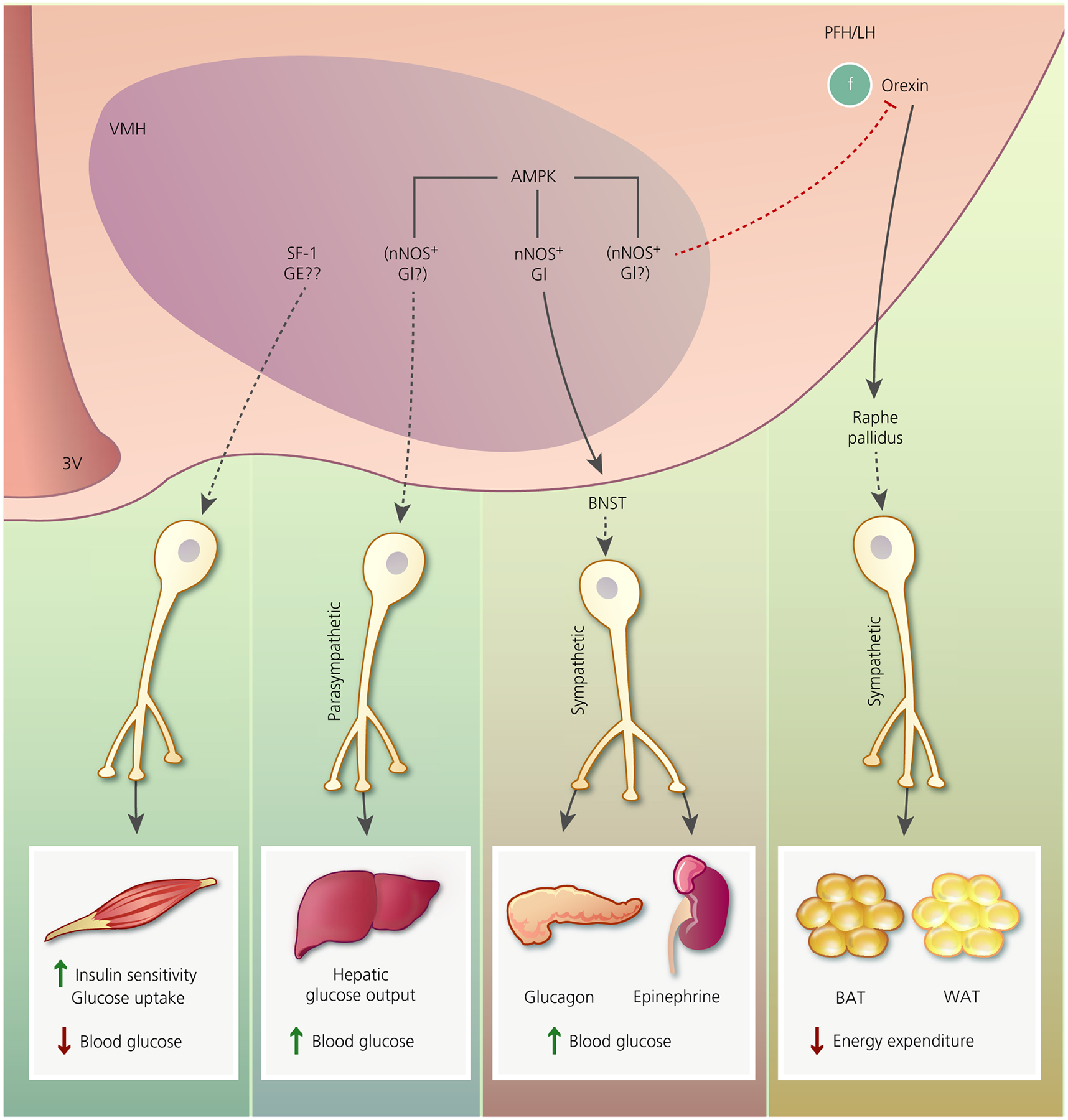
Putative pathways by which ventromedial hypothalamus (VMH) glucose-sensing neurones may regulate glucose and energy homeostasis. Activation of steroidogenic factor 1 (SF-1) neurones increases skeletal muscle insulin sensitivity and glucose uptake, leading to decreased blood glucose. We speculate that this might involve glucose-excited (GE) neurones. VMH AMP-activated protein kinase (AMPK) activation increases hepatic glucose output via a parasympathetically mediated mechanism, leading to increased blood glucose. During hypoglycaemia, VMH AMPK activation restores euglycaemia by increasing glucagon and epinephrine secretion. This effect is mediated by activation of the sympathetic nervous systems via likely projections to the bed nucleus of the stria terminalis (BNST). By contrast, VMH AMPK activation is associated with blunted brown fat (BAT) thermogenesis and white fat (WAT) browning. This effect is dependent on projections from orexin neurones in the perifornical and lateral hypothalamus (PFH/LH) to the raphe pallidus. Oestrogen activation of thermogenesis via this pathway requires VMH AMPK inhibition. VMH nitric oxide synthase (nNOS)-glucose-inhibited (GI) neurones have a demonstrated role in glucagon and epinephrine secretion during hypoglycaemia. Based on the effects of VMH AMPK described above, we speculate that VMH nNOS-GI neurones may play a role in hepatic glucose output and BAT/WAT thermogenesis. We further speculate that, because oestrogen blunts activation of VMH GI neurones by inhibiting AMPK, sGI and/or AdGI neurones may mediate some of the effects of oestrogen. 3V, third ventricle
