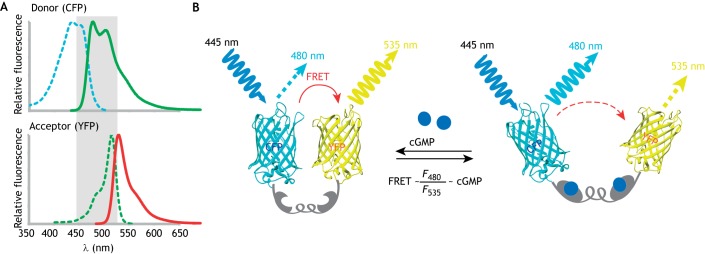
Fig. 2.
Working principles of FRET. (A) Overlap of the emission and excitation spectra of donor (CFP) and acceptor (YFP). (B) The intramolecular FRET biosensor; a cGMP biosensor is shown as an example. cGi500 (Russwurm et al., 2007) is a cGMP indicator consisting of the engineered cGMP-binding domain of the cGMP-dependent protein kinase type I (gray) sandwiched between CFP and YFP (Wen et al., 2018). The energy transfer occurs from excited CFP to YFP. Upon cGMP binding to the sensor, the FRET efficiency is reduced. Thus, light emission from YFP at 535 nm is decreased, while emission from CFP at 480 nm is increased. The ratio of emission at 480 nm and 535 nm (F480/F535) is related to the cGMP level.