Abstract
Study Objectives:
It is uncertain whether obstructive apnea (OSA) or periodic limb movements (PLMs) contribute to excessive wake time (EWT) when EWT and these disorders coexist. We hypothesized that such EWT is an independent disorder related to central regulation of sleep depth. Accordingly, we compared sleep depth in patients with EWT and OSA/PLMs (EWT+P) with patients with EWT and no OSA/PLMs (EWT-NP) and patients with a normal wake time
Methods:
A total of 267 participants were divided into five groups: (1) EWT+P: n = 100 (wake time > 20% total recording time; TRT) with OSA (apnea-hypopnea index 5–110 events/h) and/or PLMs (PLM index 10–151 events/h); (2) EWT-NP: n = 49 (wake time > 20%TRT), no associated pathology; (3) normal wake time (NWT)+P: n = 54 (wake time < 20%TRT, with OSA/PLMs); (4) NWT-NP: n = 26; (5) Healthy participants: n = 38 (no sleep complaints, NWT and no OSA/PLMs). Sleep depth was evaluated by the odds ratio product (ORP; 0 = deep sleep, 2.5 = fully alert). We also measured ORP in the 9 seconds immediately following arousals (ORP-9) to distinguish between peripheral and central mechanisms of light sleep.
Results:
ORP during sleep was higher (lighter sleep) in both EWT groups than in the three NWT groups (P < 1E-11) with no difference between those with and those without OSA/PLMs. ORP-9 was also significantly higher in the EWT groups than in the NWT groups (P < 1E-19), also with no difference between those with and without OSA/PLMs, indicating that the lighter sleep was of central origin. There were highly significant correlations between wake time and ORP-9 across all groups (P < 1E-35).
Conclusions:
EWT associated with OSA/PLMs is independent of OSA/PLMs and related to abnormal central regulation of sleep depth.
Citation:
Younes M, Giannouli E. Mechanism of excessive wake time when associated with obstructive sleep apnea or periodic limb movements. J Clin Sleep Med. 2020;16(3):389–399.
Keywords: insomnia with short sleep duration, odds ratio product, ORP-9, sleep depth
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea and periodic limb movements are commonly associated with excessive wake time. Management of such patients is complicated by uncertainty as to whether the excessive wake time is secondary to the associated pathology or is an independent disorder akin to primary insomnia with short sleep duration.
Study Impact: The current study shows that when excessive wake time coexists with sleep apnea or periodic movements it is an independent disorder related to abnormal central control of sleep depth. Although this needs direct experimental validation, these findings suggest that treatment of sleep apnea and/or periodic movements in such cases is unlikely to reduce wake time or eliminate insomnia symptoms.
INTRODUCTION
Wake time among patients undergoing clinical polysomnography (PSG) varies considerably. When, as often happens,1–5 obstructive apnea (OSA) or periodic limb movements (PLMs) are associated with excessive wake time (EWT) it is not clear whether the EWT is an independent disorder or is secondary to the associated pathology.6 Furthermore, given that easy arousability is a risk factor for OSA,7–11 it is also possible that light sleep contributes both to the EWT and to the OSA when the two coexist.
The odds ratio product (ORP) is an index derived from the relation of electroencephalography (EEG ) power in four EEG frequency ranges to each other.12 There is considerable evidence to support ORP as a continuous index of sleep depth.13 Thus, ORP correlates well with the visual appearance of the EEG,12 and it changes in the appropriate direction following sleep deprivation,14 sleep restriction,15 and across the night.16 It also responds to noise challenges that are well below levels that result in cortical arousal.17 Most importantly, in 58 patients with various sleep disorders the correlation between ORP at any given moment and likelihood of a spontaneous arousal occurring within the next 30 seconds (arousability) is almost perfect (r2 = 0.98).12 ORP ranges from 0 (very deep sleep) to 2.5 (full wakefulness).
ORP is measured every 3 seconds, making it possible to determine the time course of progression of sleep depth with high resolution. We previously found that following arousals ORP decreases rapidly, from near wake levels, for about 9 seconds. Subsequently, ORP decreases at a much slower rate.18 ORP at the inflection between the fast and slow phases (ORP-9), varies considerably among patients from 0.3 (sleep becomes very deep within seconds of the end of arousal) to 1.8, reflecting a highly arousable state in the aftermath of arousals.18 Thus, patients with high ORP-9 are more susceptible to repeat arousal and awakening, particularly when return to sleep triggers events that generate new arousal stimuli, as is the case of OSA-susceptible patients.
The objectives of the current study were to determine whether presence of OSA and/or PLMs contributes to wake time when EWT and these disorders coexist, and whether rate of sleep depth recovery following arousals (ORP-9) is correlated with wake time during clinical polysomnography regardless of associated pathology. To address these issues, we determined sleep depth, using ORP, and postarousal rate of sleep recovery (ORP-9) in patients with EWT plus OSA/PLMs and compared the results to those with EWT and no OSA/PLMs, patients referred for investigation of a sleep disorder and had normal wake time (NWT), and normal sleepers.
METHODS
We utilized PSG tests from several studies because we wished to include patients with a wide range of wake times both with and without associated pathology:
-
1.
All PSG tests (n = 161) included in two previous unrelated studies19,20 after excluding 8 studies in which continuous positive airway pressure (CPAP) was applied throughout the night. Of these (n = 153), 105 had OSA (apnea-hypopnea index [AHI] 5–110 events/h), 52 had PLM index > 10 events/h (range 10–128 events/h) of whom 42 had both disorders. Thirty-eight patients had neither disorder. By our laboratory’s protocol, all patients who demonstrate moderate/severe OSA in the first few hours are placed on CPAP (split studies). Fifty-four patients underwent split studies. Only the diagnostic portion of the study was used in this analysis.
-
2.
All patients in the database who underwent diagnostic PSG between November 2016 and October 2017 and whose discharge diagnosis included the term insomnia (n = 77). Diagnosis of insomnia was based on the latest diagnostic manuals.21,22 Forty-nine of these patients had OSA (AHI 5–50 events/h) and/or PLM index (10–151 events/h).
Patients in groups 1 and 2 had presigned a consent form, approved by our research ethics board, to permit use of their sleep record for research, or were included in previous studies approved by the Board.
-
3.
Patients in the aforementioned two groups were all referred to the sleep center for suspected sleep disorders and, hence, had sleep-related complaints. To compare the results of patients with sleep complaints/pathology with normal sleepers we identified participants in the Sleep Heart Health Study (SHHS-2)23,24 who met the following criteria: (1) total recording time (TRT) > 6.0 hours; (2) sleep efficiency > 80%; (3) AHI < 5 events/h, (4) no complaints of restless legs; (5) answered 1 (never) on each of the three primary insomnia questions (difficulty falling asleep, difficulty getting back to sleep, early awakening). Only 38 participants (from 2,645 participants in SHHS-2; 1.5%) met these criteria.
All PSG tests were manually scored when first recorded. For this study, PSG tests were scored again digitally using a validated19,20,25,26 computerized scoring system (Michele Sleep Scoring [MSS]; Cerebra Health, Winnipeg, Canada). MSS generated times in different stages, onset and end times of arousals as well as ORP values in consecutive 3-second epochs. Intraclass correlation coefficient (ICC) for agreement between non-rapid eye movement (NREM) arousal scoring by MSS and average score of 10 highly experienced scorers was previously reported at 0.83,25 whereas agreement between the 10 technologists ranged from 0.24 to 0.75 (average ICC = 0.59).25
The method of calculating ORP was described in detail elsewhere.12 Briefly, fast Fourier transform is applied to C3 and C4 EEG signals. Sum of powers is calculated in four frequency ranges; 0.33–2.33 Hz, 2.67–6.33 Hz, 7.0–14.0 Hz, and 14.3–35.0 Hz. Power in each range is assigned a rank (0–9) based on its location within the entire range encountered in clinical PSG tests. The four ranks in each 3-second epoch are combined in a 4-digit number resulting in 10,000 possible fingerprints (0000 to 9999) that describe the relative powers in the four bands. Probability of each pattern occurring during arousals or in epochs manually scored wake is determined by reference to a look-up table. Probability (0% to 100%) is divided by 40 (% of wake epochs in the development files), resulting in a range from 0 (never occurs during wakefulness or arousals) to 2.5 (never occurs during epochs scored asleep). ORP values in C3 and C4 were averaged. The 3-second ORP values were used to determine ORP-9. Average ORP in all epochs in different stages was also calculated.
Determination of rate of sleep depth recovery following cortical arousals (ORP-9)
As described previously,18 average ORP in the 9 seconds following the end of cortical arousals was calculated for all NREM arousals and these values were averaged in each PSG. ORP-9 is critically affected by the time selected by the scorer as the end of arousals. This is a highly subjective decision, particularly when arousals fade gradually into sleep. To minimize this uncertainty, in the original study a single highly experienced technologist visually identified the end of each arousal in all PSGs. This was not practical in the current study. Accordingly, in the current study, end of arousals was identified digitally by MSS using specialized algorithms.
Statistical analyses
PSGs were divided into five groups: (1) EWT with associated pathology (EWT+P): sleep efficiency (SE) < 80% plus AHI > 5 events/h, and/or PLM index > 10 events/h; (2) EWT (SE < 80%) without associated pathology (EWT-NP); (3) hospital patients with NWT (SE > 80%) with OSA/PLMs (NWT+P); (4) NWT with no OSA or PLMs; and (5) normal sleepers from SHHS-2 (NWT-SHHS). Analysis of variance and chi-square test were used to identify differences between the five groups. Where significant differences were found the t test or chi-square test was used to determine differences between individual groups with critical P = .005, allowing for 10 comparisons. ICC and Pearson correlation coefficients were calculated as needed. Results are presented either as mean ± standard deviation or mean (5th, 95th percentiles).
RESULTS
Patient characteristics
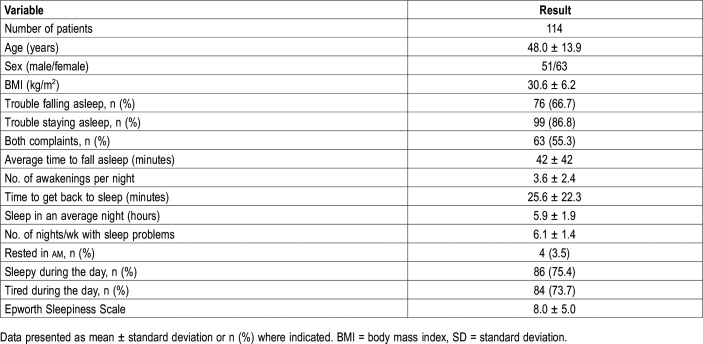
Table 1 shows demographics, conventional sleep variables, and average ORP in different sleep stages in the five groups. There were significant age differences between participants in the different groups. Of note, however, the three groups with NWT included the groups with the youngest and oldest people. Thus, the ages of patients with EWT were well within the age range in patients with NWT. Subjects with no OSA/PLMs were predominantly female, whereas the opposite was true for the other two groups. Body mass index was not significantly different among the three groups with no OSA/PLMs whereas those with OSA/PLMs had higher body mass index (Table 1). Insomnia symptoms were, by design, absent in the healthy participants from SHHS whereas they were quite prevalent in the referred patients, with no difference among the four groups.
Table 1.
Patient characteristics and polysomnography findings.
TRT was significantly lower in the OSA/PLMs groups because these two groups included split studies where the diagnostic component was only a few hours long (220 ± 68 minutes). Wake time (%TRT) was slightly higher in the two referred groups with NWT than in the participants from SHHS with no significant difference between those with and without OSA/PLMs. Average wake time in patients with EWT was approximately 40% of TRT, corresponding to an average SE of ≈60%. As may be expected, arousal/awakening index was higher in patients with OSA/PLMs than in those without.
OSA was present in 94% of patients in the NWT+P group and in 84% of patients in the EWT+P (not significant, Table 1). PLM index > 10 events/h were present in 46% and 47% of patients in the two groups with pathology (not significant, Table 1). Many patients had both disorders. Average AHI and PLM index was also not significantly different between the two pathology groups (NWT+P and EWT+P) despite the large difference in wake time (Table 1).
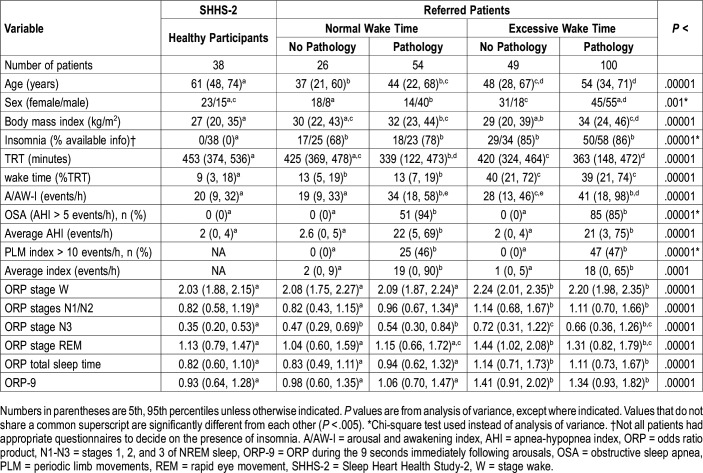
Average ORP was higher during wake time (lower sleep propensity) and in different sleep stages (lighter sleep) in the two EWT groups than in the three NWT groups. There was, however, no difference in ORP between EWT+P and EWT-NP patient groups in any sleep stage (Table 1). There was also no difference between the two referred (hospital) groups with NWT despite the marked difference in AHI and PLM index (Table 1). Interestingly, other than a slightly lower ORP in stage N3 sleep, ORP values did not differ between participants from SHHS and hospital patients with NWT also regardless of AHI or PLM index. Figure 1A shows the relation between ORP in total sleep time (TST) and wake time in all patients (n = 267). There was a highly significant correlation (r2 = .35, P < 1E-25).
Figure 1. Relationship between ORP in total sleep time and during the first 9 seconds after arousal and wake time.
(A) Relationship between average odds ratio product (ORP) in total sleep time and wake time in all patients (n = 267). Normals = normal sleepers in the Sleep Heart Health Study, NWT-NP = in hospital studies with normal wake time (NWT) and no associated pathology, NWT+P = in hospital studies with NWT and obstructive sleep apnea (OSA) and/or periodic limb movements (PLMs), EWT-NP = patients with excessive wake time (EWT; sleep efficiency < 80%) and no associated pathology, EWT+P = EWT and associated OSA/PLMs. (B) Same relation but using ORP during the first 9 seconds after arousal instead of ORP during sleep.
Dynamics of sleep depth recovery following arousals
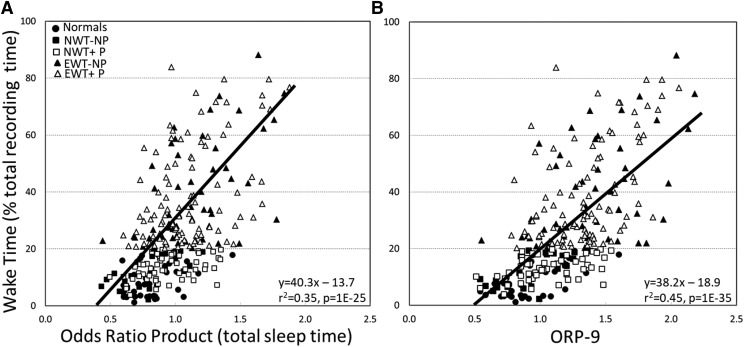
Figure 2 shows examples of the time course of ORP following a single arousal in each of two patients. The top panel is from a patient in the EWT+P group (wake time 187 minutes). The lower panel is from a patient in the NWT-NP group (wake time 29 minutes). In both cases arousal was intense with ORP rising to nearly the maximal level (≈2.5) during the arousal. In both cases there was a rapid decline in ORP following the end of arousal (dashed vertical line). In the top panel ORP did not decrease below 1.2 during this rapid phase and it fluctuated around 1.5 (transitional state) in the subsequent minute. By contrast, in the lower panel ORP decreased to < 0.5 (deep sleep) in the rapid phase and fluctuated around 0.5 in the subsequent minute. ORP-9 was 1.51 in the top panel and 0.65 in the bottom panel.
Figure 2. Contrasting postarousal sleep depth in two patients.
ORP = odds ratio product, ORP-9 = average ORP in the first 9 seconds after the end of arousal, EMG = chin electromyogram, C3/M2 = left central EEG, C4/M1 = right central EEG. End of arousal is identified by the vertical dashed line. Numbers between the two EEG tracings are the ORP values in consecutive 3-second epochs. Numbers below the pulse tracing are heart rate. Arousal was intense in both cases as indicated by a near-maximal ORP during the arousal (time 0–9 seconds). Note that the time course of ORP beyond the first 9 seconds was essentially dictated by ORP in the first 9 seconds following the arousal.
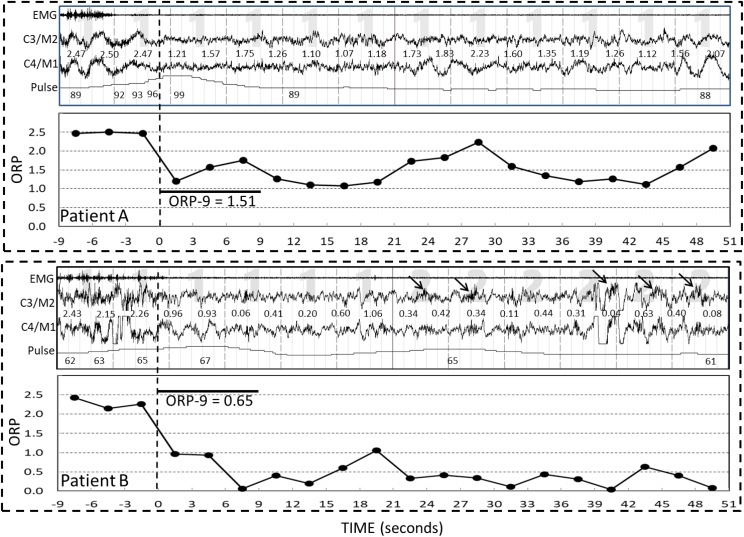
Figure 3 shows the average time course of ORP following all arousals in the two patients in Figure 2. The right axis indicates arousability, expressed as probability of a natural arousal occurring within 30 seconds at different ORP levels.12 Because the time between each arousal and the next arousal varies considerably the number of observations available for averaging decreased progressively with time (numbers adjacent to each graph).
Figure 3. Average time course of all arousals in the two patients shown in Figure 2.
Numbers above each line are number of observations averaged at the time. Thus, the number “67” above line A at 27 seconds indicates that there were 67 arousals where no subsequent arousals occurred in the subsequent 27 seconds and, accordingly, data were available for averaging. Number of observations decreases as a function of time reflecting loss of observations due to secondary arousals in the intervening periods. Bars are standard error of the mean. Right ordinate values are a measure of arousability at different odds ratio product (ORP) levels (Younes M, Ostrowski M, Soiferman M, et al. Odds ratio product of sleep electroencephalography (EEG) as a continuous measure of sleep state. Sleep. 2015;28:641–654). Note the larger number of arousals with a minimum of 9 seconds available postarousal in patient A (78 versus 25), reflecting a higher arousal index, and the slower rate of decline in the numbers of available arousals in patient B, reflecting longer interarousal intervals.
Consistent with the higher arousability in patient A, this patient had many more eligible arousals than patient B (78 versus 25) and the number of available arousals included in the average declined faster within the first postarousal minute (78→35 versus 25→19), indicating that in patient A many arousals occurred within 1 minute of a preceding arousal.
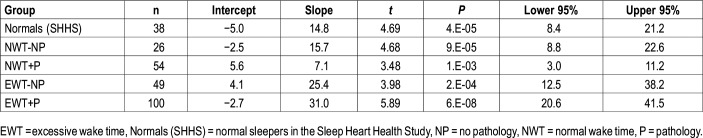
ORP-9 was significantly higher in both EWT groups than in the three NWT groups with no difference between the two EWT groups, or between the three NWT groups, despite the marked difference in AHI and PLM index. Figure 1B shows the relation between ORP-9 and wake time for all participants (n = 267). The slope was essentially the same as in Figure 3A but the intercept is shifted to a higher level indicating that, as expected,17 ORP-9 is systematically higher than ORP during sleep. The regression equation indicated that 45% of the variance in wake time could be explained by differences in ORP-9. Although the relation in Figure 1B appears nonlinear, an exponential fit yielded the same coefficient as the linear fit (0.67 versus 0.67). Table 2 shows that the confidence intervals of the slopes in the two EWT groups (with and without pathology) essentially overlap completely and the slopes in the three NWT groups also substantially overlap. The two slopes in the EWT groups are higher than the three NWT groups but their confidence intervals overlap with those of the three NWT slopes.
Table 2.
Regression equations of ORP-9 versus wake time (% total recording time) in the five groups.
Determinants of wake time
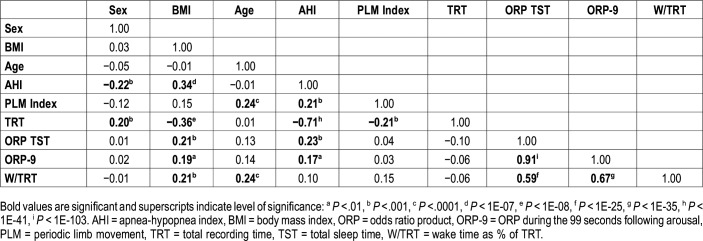
Table 3 shows results of univariate regression analysis that included variables potentially relevant to wake time. By far, the strongest correlations with wake time were with ORP-TST (r = .59, P < 1E-25) and ORP-9 (r = .67, P < 1E-35) and, as reported previously,18 these two variables were highly correlated (r = .91, P < 1E-103). Weaker correlations were found with age and body mass index (Table 2). There was no significant correlation with sex, AHI, PLM index, or TRT. A highly significant correlation was found between AHI and TRT (r = −.71, P < 1E-41). However, this is an experimental artifact because results from PSG tests with very short TRT were derived from the split studies, which invariably belonged to patients with high AHI.
Table 3.
Correlation matrix of variables used in multiple regression analysis.
In multiple linear regression analysis with backward elimination, using the same variables listed in Table 2, the only significant correlates with wake time were: ORP-9 (P < 1E-35), age (P < .01), and the PLM index (P < .05). The final multiple regression equation (r = .69, P < 1E-37) was:
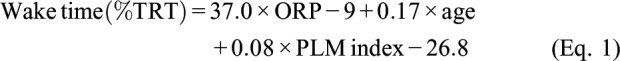 |
Using ORP-TST instead of ORP-9 yielded similar results (r = .61, P < 1E-27):
Relation between insomnia symptoms, ORP, and associated pathology
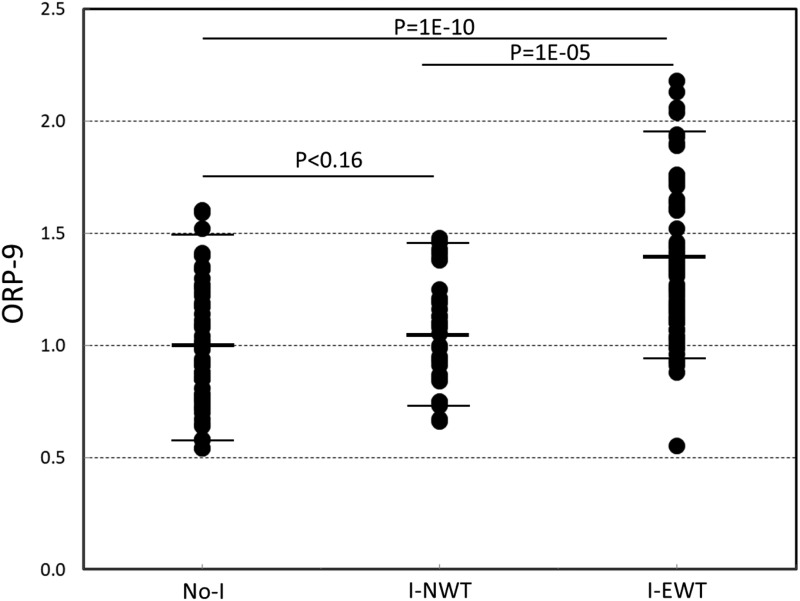
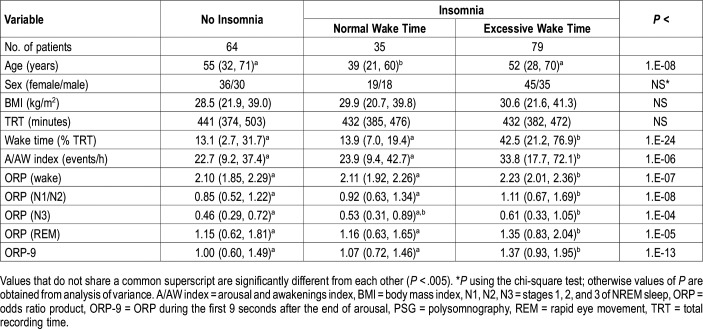
Custom questionnaires used in our laboratory were available in 183 patients. These questionnaires included several questions pertinent to insomnia. Based on these questionnaires, a total of 114 patients met the latest criteria21,22 of an insomnia disorder, which allow diagnosing the disorder even when OSA/PLMs coexist in the same patient (Table 1). The self-reported complaints of these patients are outlined in Table 4. Of these, 79 had EWT (42.5 ± 19.2%TRT) and 35 had NWT (13.9 ± 4.4%TRT) (Table 4). Sixty-four patients had no insomnia disorder with a wide range of wake times (13.1 ± 10.9%TRT). Patients with I-EWT had significantly more arousals and awakenings than the other two groups (Table 4). Wake ORP, NREM ORP, and rapid eye movement ORP were all significantly higher in the I-EWT group than in the other groups, whereas there was no significant difference between No-I and I-NWT. Figure 4 shows the distribution of ORP-9 in the three groups. Average ORP-9 was markedly higher in I-EWT than in the other groups (P = 1E-13, Table 4). In 22 I-EWT patients ORP-9 was completely outside the range found in I-NWT and No-I.
Table 4.
Characteristics of patients with a clinical diagnosis of insomnia.
Figure 4. Range of odds ratio product (ORP) in the first 9 seconds after arousal (ORP-9) in patients with no insomnia (No-I) and insomnia patients with normal (I-NWT) and excessive (I-EWT) wake time.
Horizontal bars are means and 5 and 95 percentiles.
We determined the incidence of insomnia symptoms at different AHI quartiles in the four hospital groups (participants from SHHS were excluded from this analysis as absence of insomnia symptoms was a precondition for inclusion in this study). There was no difference in incidence between the lowest quartile (AHI 0.7 events/h, range 0.0–2.6 events/h; incidence 30/36) and the highest quartile (AHI 18.7 events/h, range 12.3–49.6 events/h; incidence 30/36). Likewise, there was no difference in insomnia incidence as a function of PLM index (32/36 in the lowest quartile, PLM index = 0, and 29/36 in the highest quartile, PLM index 31.2 events/h, range 11–151 events/h).
DISCUSSION
The main findings from this study are that when EWT and OSA/PLMs coexist in clinical PSG the presence and extent of wake time as well as presence of insomnia symptoms appear to be independent of the associated pathology, and wake time is highly correlated with sleep depth immediately after arousals (ORP-9).
Association of EWT and sleep apnea and/or PLMs
The combination of EWT and OSA/PLMs is very common. Sleep efficiency is approximately 80 ± 12% in patients with OSA1–3 and in those with PLMs.4,5 The relation between EWT and associated OSA/PLMs is unclear.6 The following possibilities exist: (1) the association reflects chance occurrence of common but independent abnormalities; (2) EWT is a consequence of frequent awakenings produced by the associated pathology27; this possibility is the basis for treating OSA in such cases with the expectation of relieving the insomnia; and (3) the association is produced by an abnormality in sleep regulation that results in light sleep because light sleep (high beta activity) is a feature of insomnia with short sleep duration28–30 and is also a risk factor for OSA.7–11 The uncertainty about the nature of this association has important diagnostic and therapeutic implications.6
We addressed this uncertainty by determining the relation between wake time and sleep depth (ORP). ORP increases if beta power is higher and/or if delta/theta powers are lower.12 Significant positive correlation between ORP and wake time would support the possibility that light sleep is contributing both to EWT and, possibly, to OSA (as previously discussed).
Sleep depth was substantially lighter in the two groups with EWT than in groups with NWT (Table 1). In addition, there were no significant differences in sleep depth between those with and without pathology whether wake time was normal or excessive. ORP-9 emerged as the most significant correlate with wake time whereas OSA and PLM severities had minimal effect on wake time (Equation 1 and Equation 2). These results strongly suggest that wake time is independent of any associated OSA and minimally related to PLMs. This conclusion is supported by a recent study on the effect of CPAP on sleep in patients with combined OSA and insomnia.31 In this study, objective sleep time, sleep latency, wake after sleep onset, and sleep efficiency changed little during CPAP therapy.31
ORP-9 and regulation of sleep depth
We showed previously,18 and confirmed again in this study, that sleep depth (average ORP during sleep, ORP-TST) is highly correlated with the depth reached within the first 9 seconds following cortical arousals (ORP-9; r = .91, Table 3) and that ORP-9 is highly variable among participants (Table 1). Beyond 9 seconds ORP decreases very slowly and only if no subsequent arousals occur (Figure 3). Because a high ORP-9 increases the likelihood of arousals and awakenings occurring soon after a previous arousal, the likelihood of the patient with high ORP-9 not developing another arousal soon after a previous one is small. The repeated interruption of the slow declining phase makes it difficult to reach deep sleep. When patients with severe OSA and high ORP-9 are placed on CPAP in the same night (split studies) marked reduction in the arousal/awakening index is associated with lower sleep ORP during therapy on account of the longer interarousal interval. But ORP-9 does not change.18 This indicates that ORP-9 is determined by central mechanisms that regulate sleep depth and is independent of prevailing sleep fragmentation whereas ORP during sleep is affected both by ORP-9 and the profile of arousal stimuli. ORP-9 is therefore useful in determining whether light sleep is due to excessive arousal stimuli (eg, somatic, environmental) or to abnormalities in central regulation of sleep depth.
The mechanisms responsible for differences in ORP-9 among individuals are unknown. It is possible that a higher frequency and intensity of peripheral arousal stimuli accounts for these differences. However, for this to be the case, stimuli would have to occur with sufficiently high frequency as to arrest sleep progression within 9 seconds of the end of almost every arousal (note the very small standard error at 9 seconds in Figure 3). With just one stimulus every 9 seconds, frequency of these stimuli must exceed 300 times/h. Although this is possible, several preliminary observations suggest that ORP-9 is a trait: (1) Except for patients with insomnia and EWT, where the range is wider (Figure 4), the range of ORP-9 is the same (0.4–1.7) in young14,15 and old individuals32 whether healthy or with sleep disorders.18 (2) ORP-9 is fairly reproducible across days, weeks, and years (ICC ≈ 0.60).14,33 (3) Although ORP-9 decreases following sleep deprivation and restriction,14,15 the decrease (≈ –0.2 following 38 hours of total sleep deprivation)14 is a small fraction of the entire ORP-9 range among individuals (0.4–1.7).
ORP-9 was substantially lower in the NWT groups than in patients with EWT and not different between those with and without pathology (Table 1). We therefore conclude that the lighter sleep in the patients with EWT is likely related to an abnormality in central regulation of sleep depth. That differences in central regulation of sleep depth, as reflected by the rate of sleep recovery following arousals (ORP-9), are primary whereas differences in ORP-TST, which are influenced in addition by extent of sleep fragmentation, are secondary is supported by the fact that the correlation between wake time and ORP-9 (r2 = .45, P = 1E-35) was stronger than the correlation between wake time and ORP-TST (r2 = .35, P = 1E-25, Figure 1).
Relation between ORP-9 and the arousal threshold
This relation was described in detail earlier.13,16,18 Briefly, we have shown that current ORP is highly correlated with the likelihood of a spontaneous arousal occurring within 30 seconds (r2 = .98).12 Thus, analogous to the arousal threshold, current ORP reflects the ease with which an arousal stimulus can trigger a cortical arousal. ORP-9 determines the highest ORP that may exist in NREM sleep. Because in the absence of arousals ORP continues to decrease monotonically until deep sleep is reached,18 actual ORP, and hence arousability/arousal threshold, at any time is a function of ORP-9 and the interval between successive arousals (Figure 3). The range over which NREM ORP can vary is large in individuals with high ORP-9, and vice versa. Subjects with high ORP-9 can reach deep sleep only if their arousal stimuli are weak and infrequent whereas patients with low ORP-9 will enjoy deep sleep with less arousals unless the arousal stimuli are very strong and frequent.
Relevance to the insomnia disorder
Insomnia symptoms are common (40% to 50%) in patients with OSA.27,34 Until recently, insomnia associated with OSA or PLMs was considered a separate entity and included in the “secondary” or “comorbid” insomnia category.35,36 In 2005 a National Institutes of Health panel expressed concern about this separation from primary insomnia given the lack of direct evidence that the associated pathology was causing the insomnia.6 As a result, the latest versions of the diagnostic manuals do not distinguish between insomnia with and without associated pathology; both kinds are called “Chronic Insomnia Disorder.”21,22 However, the possibility that associated pathology is contributing to insomnia in such cases remained.
By showing that incidence of insomnia is independent of presence (Table 1, four referred patient groups) or severity (quartile analysis) of associated OSA and PLMs our findings suggest that when insomnia and OSA/PLMs coexist the coexistent pathology is not responsible for the presence of insomnia symptoms. Interestingly, in a very recent study, Sweetman et al31 found that insomnia symptoms improved significantly on CPAP in insomnia patients with comorbid OSA. However, the Insomnia Severity Index (ISI) remained elevated after 6 months of CPAP (11.6, 95% confidence interval: 10.2–12.8) indicating that in almost all patients ISI remained above the threshold for a diagnosis of insomnia (ISI > 8). Thus, insomnia was improved but not eliminated. The improvement in insomnia symptoms despite lack of change in wake time31 suggests that insomnia symptoms are sensitive to sleep depth more so than to wake time. A recent study found that NREM ORP improved (decreased) during CPAP therapy in patients with OSA.37 It would be of interest to find out if NREM ORP decreases during CPAP therapy in patients with insomnia and comorbid OSA.
Patients in the two EWT groups who complained of insomnia would ordinarily be classified as having insomnia with short sleep duration (79 of 92 with available questionnaires, Table 1). Insomnia with short sleep duration is believed to result from a hyperarousal state that interferes with sleep onset and maintenance.28,38–40 One of the advantages of ORP is that it can distinguish between different levels of “wakefulness” as the EEG transitions from full wakefulness (ORP = 2.5) to just before definite sleep (ORP = 1.5–2.0).12 ORP was higher during wakefulness in patients with insomnia and EWT than in patients with no insomnia or with insomnia and NWT (Table 5). This finding is consistent with hyperarousal as it indicates heightened vigilance during wakefulness. Furthermore, ORP was higher in all sleep stages (Table 5), consistent with sleep being lighter in patients with hyperarousal.28–30 Sleep depth recovery following arousals was slower (higher ORP-9) in patients with insomnia and EWT (Figure 4, Table 5). Because ORP-9 is the main determinant of average sleep depth,18 it is possible that slowing of rate of sleep progression is the central mechanism by which hyperarousal exerts its influence on wake time and sleep depth.
Table 5.
Patient characteristics and polysomnography findings according to insomnia type.
Limitations
Age differed among the five patient groups (Table 1). Although age was correlated with wake time (Table 2 and Equation 1 and Equation 2) the effect of age is very small by comparison to differences in wake time among the groups. Thus, age coefficient was ≈ 0.2%TRT per year (Equation 1 and Equation 2). An age difference of 20 years would account for 4% difference in wake time (%TRT) whereas differences in wake time between groups with and without EWT were substantially larger (Table 1).
The two groups with pathology (NWT+P and EWT+P) included 54 patients who underwent split studies and in whom analysis was limited to the pre-CPAP portion of the PSG. It may be argued that the difference in TRT between those with and without pathology may have influenced the results. However, neither ORP-9 nor ORP-TST correlated with TRT (Table 3). Furthermore, we recalculated ORP values after excluding the split studies and there were no changes in ORP-9 or ORP in sleep stages, and no changes in the results of statistical comparisons between groups. For example, average ORP-9 changed from 1.06 (Table 1) to 1.07 in the NWT+P and from 1.34 to 1.33 in the EWT+P.
There were clearly important technical differences between participants in the SHHS study and the referred patients. These include different acquisition equipment, home versus laboratory environment, and no sleep complaints versus sleep complaints. ORP is, however, insensitive to acquisition equipment so long as the signals are acquired according to the recommended calibrations, filters and electrode locations, and they were. Differences in the environment and sleep complaints may have affected ORP. However, we found no differences in ORP values or in arousal/awakening index between the participants in the SHHS study and referred patients with NWT and no pathology (Table 1). It would be very unlikely that this lack of difference results from opposing influences of environment and sleep complaints on sleep depth. Thus, we think that lack of difference in sleep depth between these two groups suggests that differences between home and laboratory environment and presence of sleep symptoms in the absence of sleep pathology or EWT do not materially alter sleep depth when the individual is asleep.
CONCLUSIONS
The main conclusion from this study is that when EWT is present in patients with mild/moderate OSA or PLMs the EWT is unrelated to the associated pathology. Rather, wake time appears to be most correlated with the central regulation of sleep depth progression (ORP-9). Furthermore, a slow rate of sleep progression following arousals may be a mechanism by which the hyperarousal state effects the increase in wake time in patients with insomnia and short sleep duration.
ACKNOWLEDGMENTS
The authors thank Wayne Thompson, RPSGT, for assistance in collecting the polysomnograms and related documents. The Sleep Heart Health Study sleep records were provided by the National Sleep Research Resource (NSRR). The NSRR is supported by Grant Number HL114473 from the National Heart, Lung, and Blood Institute, NIH.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- EEG
electroencephalogram
- EWT
excessive wake time
- EWT+P
excessive wake time associated with OSA or PLMs
- EWT-NP
excessive wake time not associated with OSA or PLMs
- ICC
intraclass correlation coefficient
- MSS
Michele Sleep Scoring system
- No-I
no insomnia
- NREM
non-rapid eye movement
- NWT
normal wake time
- NWT+P
normal wake time associated with OSA or PLMs
- NWT-NP
normal wake time not associated with OSA or PLMs
- ORP
odds ratio product
- ORP-9
ORP measured during the 9-seconds immediately following arousals
- OSA
obstructive sleep apnea
- PLM
periodic limb movement
- PSG
polysomnography
- SHHS
Sleep Heart Health Study
- TRT
total recording time
- TST
total sleep time
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. EG reports no conflicts of interest. MY is the developer of the ORP method of assessing sleep depth. The technology is licensed to Cerebra Health (https://cerebrahealth.com/). MY receives royalties and consultation fees from, and is a shareholder in Cerebra Health.
REFERENCES
- 1.Mediano O, Barceló A, de la Peña M, Gozal D, Agustí A, Barbé F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J. 2007;30(1):110–113. doi: 10.1183/09031936.00009506. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 3.Seneviratne U, Puvanendran K. Excessive daytime sleepiness in obstructive sleep apnea: prevalence, severity, and predictors. Sleep Med. 2004;5(4):339–343. doi: 10.1016/j.sleep.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Claman DM, Redline S, Blackwell T, et al. Prevalence and correlates of periodic limb movements in older women. J Clin Sleep Med. 2006;2(4):438–445. [PubMed] [Google Scholar]
- 5.Claman DM, Ewing SK, Redline S, Ancoli-Israel S, Cauley JA, Stone KL. Periodic leg movements are associated with reduced sleep quality in older men. J Clin Sleep Med. 2013;9(11):1109–1117. doi: 10.5664/jcsm.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NIH State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1–30. [PubMed] [Google Scholar]
- 7.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5(6):519–524. [PMC free article] [PubMed] [Google Scholar]
- 8.Dingli K, Fietze I, Assimakopoulos T, Quispe-Bravo S, Witt C, Douglas NJ. Arousability in sleep apnoea/hypopnoea syndrome patients. Eur Respir J. 2002;20(3):733–740. doi: 10.1183/09031936.02.00262002. [DOI] [PubMed] [Google Scholar]
- 9.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105(5):1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 10.White DP, Younes MK. Obstructive sleep apnea. American Physiological Society. Compr Physiol. 2012;2:2541–2594. doi: 10.1002/cphy.c110064. [DOI] [PubMed] [Google Scholar]
- 11.Eckert DJ, Younes M. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985) 2014;116(3):302–313. doi: 10.1152/japplphysiol.00649.2013. [DOI] [PubMed] [Google Scholar]
- 12.Younes M, Ostrowski M, Soiferman M, et al. Odds ratio product of sleep EEG as a continuous measure of sleep state. Sleep. 2015;38(4):641–654. doi: 10.5665/sleep.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younes M. The case for using digital EEG analysis in clinical sleep medicine. Sleep Sci Pract. 2017;1:2. [Google Scholar]
- 14.Kuna ST, Tanayapong P, Maislin G, et al. Odds ratio product: A measure of sleep homeostasis following prolonged wakefulness. Sleep. 2018;41(suppl_1):A83. [Google Scholar]
- 15.Schweitzer PK, Griffin KS, Younes M, Walsh JK. Assessment of sleep depth and propensity during sleep restriction using the odds ratio Product. Sleep. 2018;41(suppl_1):A57–A58. [Google Scholar]
- 16.Qanash S, Giannouli E, Younes M. Assessment of intervention-related changes in non-rapid-eye-movement sleep depth: importance of sleep depth changes within stage 2. Sleep Med. 2017;40:84–93. doi: 10.1016/j.sleep.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Smith MG, Younes M, Aeschbach D, Müller U, Basner M. Planes, trains and automobiles: traffic noise and its impact on sleep depth measured by the odds ratio product. Sleep. 2019;42(suppl_1):A54. [Google Scholar]
- 18.Younes M, Hanly PJ. Immediate post-arousal sleep dynamics: an important determinant of sleep stability in obstructive sleep apnea. J Appl Physiol. 2016;120(7):801–808. doi: 10.1152/japplphysiol.00880.2015. [DOI] [PubMed] [Google Scholar]
- 19.Younes M, Thompson W, Leslie C, Egan T, Giannouli E. Utility of technologist editing of polysomnography scoring performed by a validated automatic system. Ann Am Thorac Soc. 2015;12(8):1206–1218. doi: 10.1513/AnnalsATS.201411-512OC. [DOI] [PubMed] [Google Scholar]
- 20.Younes M, Soiferman M, Thompson W, Giannouli E. Performance of a new portable wireless sleep monitor. J Clin Sleep Med. 2017;13(2):245–258. doi: 10.5664/jcsm.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 22.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 23.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 24.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 25.Malhotra A, Younes M, Kuna ST, et al. Performance of an automated polysomnography scoring system versus computer-assisted manual scoring. Sleep. 2013;36(4):573–582. doi: 10.5665/sleep.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younes M, Younes M, Giannouli E. Accuracy of automatic polysomnography scoring using frontal electrodes. J Clin Sleep Med. 2016;12(5):735–746. doi: 10.5664/jcsm.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krakow B, Melendrez D, Ferreira E, et al. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest. 2001;120(6):1923–1929. doi: 10.1378/chest.120.6.1923. [DOI] [PubMed] [Google Scholar]
- 28.Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–558. doi: 10.1016/S1474-4422(15)00021-6. [DOI] [PubMed] [Google Scholar]
- 29.Spiegelhalder K, Regen W, Feige B, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91(3):329–333. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Corsi-Cabrera M, Figueredo-Rodríguez P, del Río-Portilla Y, Sánchez-Romero J, Galán L, Bosch-Bayard J. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep. 2012;35(4):501–511. doi: 10.5665/sleep.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with co-morbid insomnia: A randomized clinical trial. Sleep. 2019;42(12) doi: 10.1093/sleep/zsz178. [DOI] [PubMed] [Google Scholar]
- 32.Stone KL, Peters KE, Redline S, et al. Novel quantitative EEG exposures and risk of incident MCI and dementia in older women. Sleep. 2018;41(suppl_1):A376. [Google Scholar]
- 33.Younes M, Mazzotti D, Redline S. Range and reproducibility of EEG biomarkers for cognition across five years. Sleep. 2019;42(suppl_1):A386–A387. [Google Scholar]
- 34.Smith S, Sullivan K, Hopkins W, Douglas J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS) Sleep Med. 2004;5(5):449–456. doi: 10.1016/j.sleep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 36.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 37.Penner CG, Gerardy B, Ryan R, Williams M. The Odds Ratio Product (an objective sleep depth measure): normal values, repeatability, and change with CPAP in patients with OSA. J Clin Sleep Med. 2019;15(8):1155–1163. doi: 10.5664/jcsm.7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnet MH, Burton GG, Arand DL. Physiological and medical findings in insomnia: implications for diagnosis and care. Sleep Med Rev. 2014;18(2):111–122. doi: 10.1016/j.smrv.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Perlis ML, Ellis JG, Kloss JD, Riemann DW. Etiology and Pathophysiology of Insomnia. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. New York, NY: Elsevier Academic Press; 2016:769-784. [Google Scholar]
- 40.Kay DB, Buysse DJ. Hyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studies. Brain Sci. 2017;7(3):23. doi: 10.3390/brainsci7030023. [DOI] [PMC free article] [PubMed] [Google Scholar]