Figure 4.
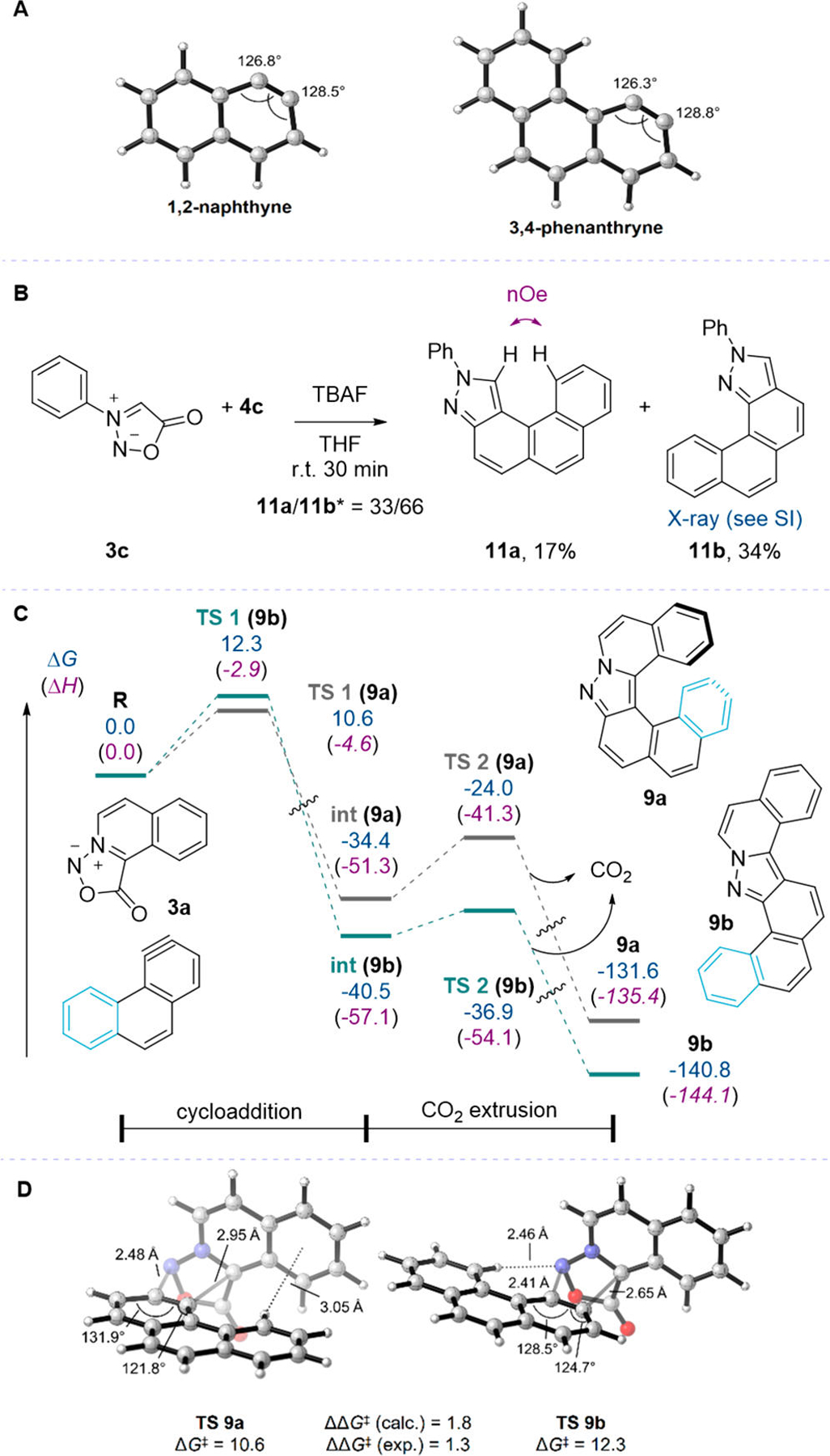
(A) DFT-optimized structures of polycyclic arynes derived from 4b and 4c. (B) Reaction between nonplanar N-phenyl sydnone 3c and 3,4-phenanthryne precursor 4c. * Isomer ratio was measured by 1H NMR of the crude mixture. (C) Energy profile for the reaction of 3a with 3,4-phenanthryne to form 9a or 9b. Free energies (enthalpies) are in kcal/mol and were obtained at the M06–2X/6–311+G(2d,2p)/SMD(THF)//M06–2X/6–31+G(d,p) level of theory. (D) Cycloaddition TSs leading to regioisomers 9a and 9b. M06–2X/6–311+G(2d,2p)/SMD(THF)//M06–2X/6–31+G(d,p). Free energies in kcal/mol.
