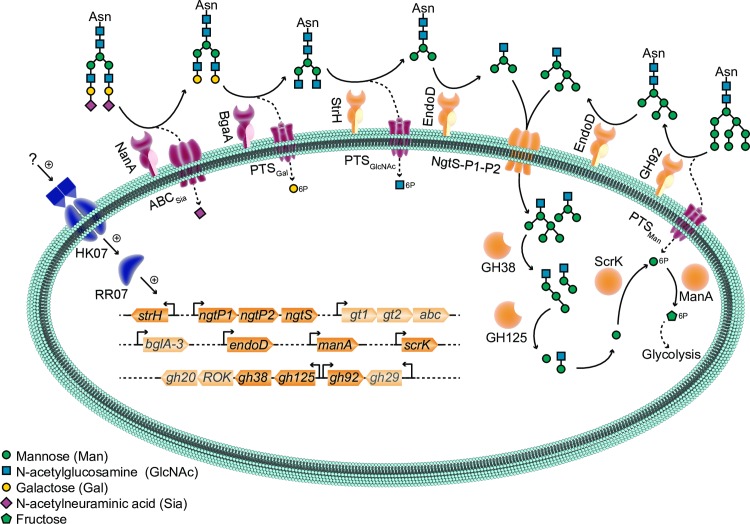
Fig 9. Model of N-glycan metabolism and TCS07 regulation.
N-glycans are sequentially cleaved by extracellular and intracellular glycoside hydrolases, most of which are transcriptionally activated by TCS07 (HK07 and RR07). Genes upregulated by TCS07 and corresponding protein products are colored yellow. First step of degrading complex N-glycan structures is cleavage of sialic acid and galactose by NanA and BgaA, respectively, neither of which are part of the identified TCS07 regulon. All other enzymatic activities related to N-glycan metabolism can be attributed to protein products of TCS07 regulated genes as follows: GlcNAc exposed by NanA and BgaA is cleaved by StrH, and the resulting Man3GlcNAc2-core is cleaved between the GlcNAc moieties by EndoD. For high-mannose N-glycans, the Man6-9GlcNAc2 is trimmed by GH92 to Man5GlcNAc2, which is cleaved by EndoD to Man5GlcNAc. The released Man3GlcNAc and Man5GlcNAc are imported by the NgtS-P1-P2 ABC transporter system. Intracellularly GH38 hydrolyze the 1,3-glycoside bonds, and GH125 hydrolyze the 1,6-glycoside bonds of the Man3GlcNAc and Man5GlcNAc resulting in ManGlcNAc. Mannose released by GH38 and GH125 is possibly phosphorylated by ScrK into Mannose-6-phosphate, which is converted by ManA to fructose-6-phosphate. Finally, fructose-6-phosphate enters glycolysis. Genes that are faded are genes that has not been attributed a function in the model. Genes marked with * are genes that were not considered upregulated based on criteria defined in Fig 1 but are part of an operon with genes considered upregulated.