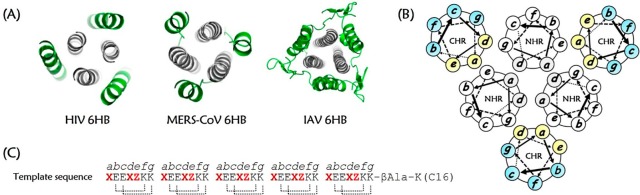
Figure 1.
Six-helix bundle (6HB) fusion core structure and the design of a lipopeptide template based on the interaction between the NHR and CHR domains. (A) Cartoon representations of the HIV (PDB 1AIK), MERS-CoV (PDB 4NJL), and influenza H3N2 (PDB 1QU1) 6HBs, in which the NHR trimers and CHR segments are colored in gray and green, respectively. (B) Helical wheel representation of a 6HB. The residues at the a, d, and e positions (yellow) form the buried face that interacts with the NHR trimers, while those at the b, c, f, and g positions (blue) are solvent-accessible sites. (C) The de novo designed lipopeptide template, in which the critical residues at the a, d, and e positions are highlighted in red font. The dotted lines show the predicted intramolecular salt bridges formed by the acidic amino acids at the i positions and the basic residues at the i + 4 positions.