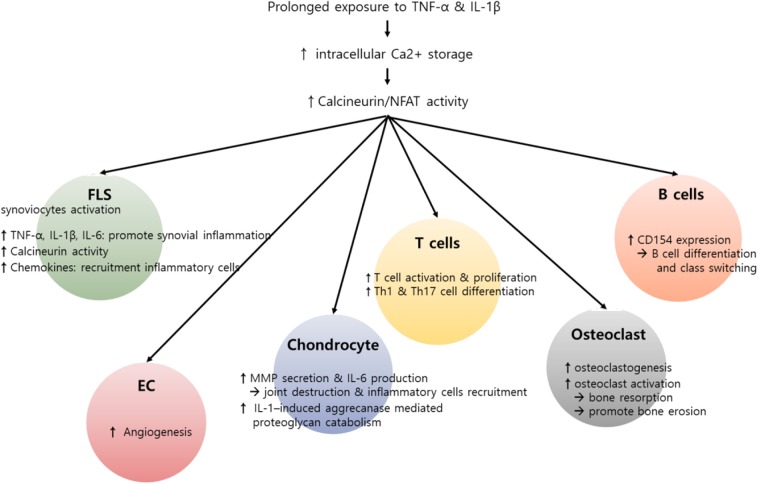
Figure 2.
Potential role of calcium (Ca2+)–calcineurin–nuclear factor of an activated T cell (NFAT) pathway in innate and adaptive immune cells involving rheumatoid arthritis (RA) pathogenesis. RA is characterized by infiltration of various inflammatory cells such as macrophages, T cells, and B cells, in addition to hyperactivation and proliferation of fibroblast-like synoviocytes (FLSs). Pro-inflammatory cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), can induce an increase in intracellular Ca2+ concentration. Subsequently, activated calcineurin propagates a wide range of signals essential to the hyperactivation of diverse immune cells involving RA pathogenesis. In T cells, activated calcineurin promotes NFAT and nuclear factor κB (NF-κB) and decisively controls T cell function, growth, and apoptosis. The calcineurin–NFAT axis can also upegulate CD154 transcription, thereby inducing B cell differentiation and antibody production. Beyond its role in lymphocytes, Ca2+-calcineurin–NFAT seems to directly activate innate and matrix metalloproteinases. Calcineurin–NFAT signaling mediates vascular endothelial growth factor (VEGF)-induced endothelial migration and proliferation and thereby can enhance pathologic angiogenesis in RA synovia (88, 89). Additionally, the receptor activator of nuclear factor-κB ligand (RANKL)-induced increase in intracellular Ca2+ levels triggers the Ca2+-calcineurin pathway in osteoclasts, which promotes osteoclastogenesis through NFATc1 activation. Taken together, dysregulated intracellular Ca2+ store and Ca2+ response contribute to the pathogenesis of RA by activating calcineurin–NFAT pathway in multiple types of cells.