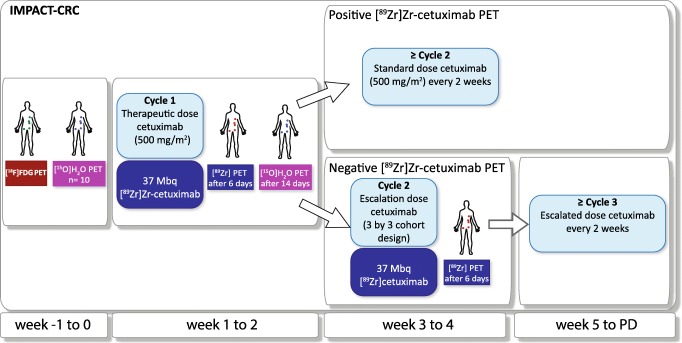
Fig. 1.
Study design of the IMPACT-CRC study. Before the first cetuximab cycle a [18F]FDG PET/CT was performed, and in a subgroup a [15O]H2O PET/CT was performed. Directly after administration of the first treatment dose of cetuximab (500 mg/m2), 37 MBq/10 mg [89Zr]Zr-cetuximab was injected and 6 days p.i. a PET/CT was performed. If there was visual uptake in at least one extra-hepatic lesion, the [89Zr]Zr-cetuximab PET/CT was considered positive and patients were treated with the standard dose every other week. In case of a negative PET/CT scan, cetuximab dose was escalated in a 3 × 3 cohort design and [89Zr]Zr-cetuximab PET/CT was repeated. These patients were treated with the escalated dose every other week. In an additional four patients an extra [89Zr]Zr-cetuximab PET/CT with low pre-dose (100 mg unlabeled cetuximab) was performed 14 days prior to treatment cycle 1 to explore the possible occurrence of saturation at a 500 mg/m2 dose (not indicated in this figure). These 4 patients underwent a second [89Zr]Zr-cetuximab tracer administration and [89Zr]Zr-cetuximab PET/CT after administration of the first treatment dose and continued with standard dose cetuximab every other week