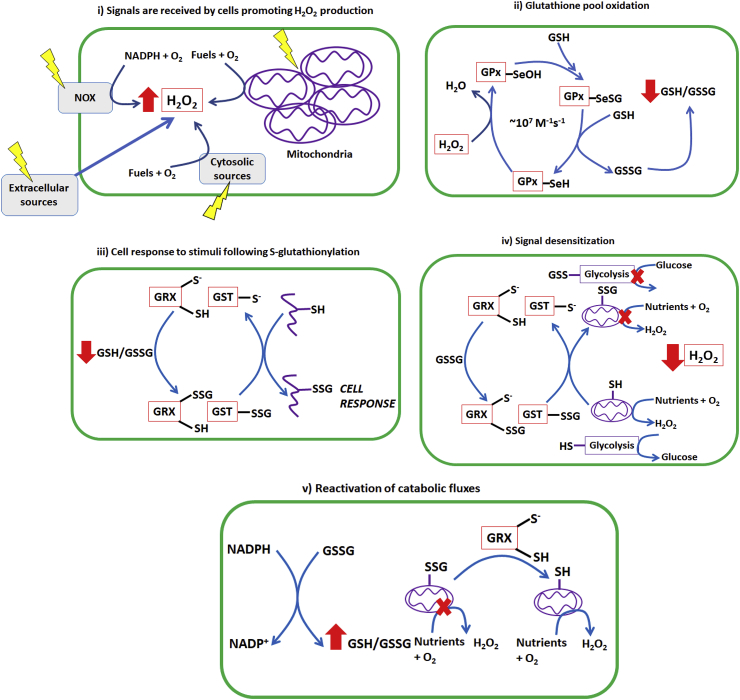
Fig. 1.
A unifying hypothesis for H2O2 second messaging through cellular glutathione pools. I) An intra- or extracellular environmental cue is received by the cell resulting in a temporary burst in H2O2 production. The source of H2O2 can also originate from adjacent cells or extracellular generators. The stimuli can induce the site-specific generation of H2O2 by extracellular, cytosolic, or mitochondrial sources. Additionally, the H2O2 signals can self-amplify one another, either through the temporary inhibition of cellular peroxiredoxins or via the propagation of ROS production through the stimulation of another generator. II) H2O2 is rapidly quenched near its source of production by the rapid action of glutathione peroxidases (GPx). Removal involves an active site selenocysteine, which is oxidized by H2O2 to form a selenol that is resolved by two reduced glutathione (GSH) molecules. This results in production of glutathione disulfide and the oxidation of the cellular glutathione pool (decrease in the ratio of GSH/GSSG). III) Decrease in GSH/GSSG is sensed by glutaredoxins (GRX1; cytosol and intermembrane space, GRX2; matrix) and glutathione S-transferase (GSTP) resulting in the S-glutathionylation of a protein resulting in information transmission. IV) Metabolic enzymes involved in glycolysis, the Krebs cycle, fatty acid and amino acid oxidation, and oxidative phosphorylation are also subjected to S-glutathionylation by GSTP and GRX1/2. This event inhibits catabolic pathways decreasing ROS production and promoting the diversion of metabolites towards pathways that synthesize NADPH. V) Provision of NADPH and inhibition of ROS production results in the reduction of glutathione pools and the restoration of cell redox buffering capacities. This activates the deglutathionylase activities of the glutaredoxins restoring metabolic fluxes through catabolic pathways.