Figure 1.
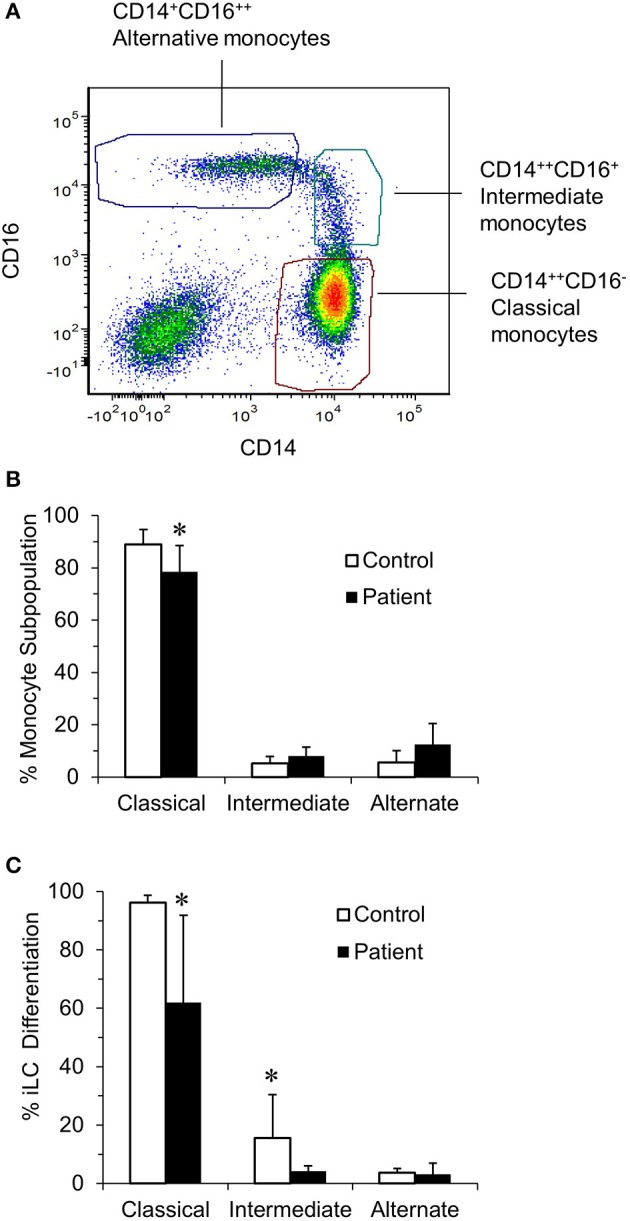
Identification of blood monocyte subsets in RRP patients and controls by flow cytometry. (A) Illustrates the characteristic of monocyte subsets from controls when immunostained with anti–CD14-PE and anti–CD16-Pacific Blue and sorted into CD14++CD16− (classical), CD14++CD16+ (intermediate), and CD14−CD16++ (non-classical) subpopulations. (B) The percentage of classical, intermediate, and non-classical (alternate) monocytes in the controls (n = 8) and patients (n = 7) determined using a BD FACSAria cell sorter. There was a significant difference in the percentage of classical monocytes between patients and controls (P < 0.05). (C) Ability of monocyte subpopulations to generate iLCs; subpopulations of monocytes from control (n = 4) and patients (n = 6) were isolated and cultured for 7 days in complete RPMI medium supplemented with 100 ng/mL GMCSF, 20 ng/mL IL-4, and 10 ng/mL TGFβ1. On day 7, cells were harvested, surface stained with fluorochrome-labeled anti–CD1a-FITC and anti–E-cadherin-APC, and then analyzed by flow cytometry. There was a significant difference in the ability of classical monocytes from patients and controls to generate iLCs (p < 0.05). Results are shown as mean ± SD; data were analyzed by one-way ANOVA for (B) and two-tailed unpaired t-test for (C). *p < 0.05.
