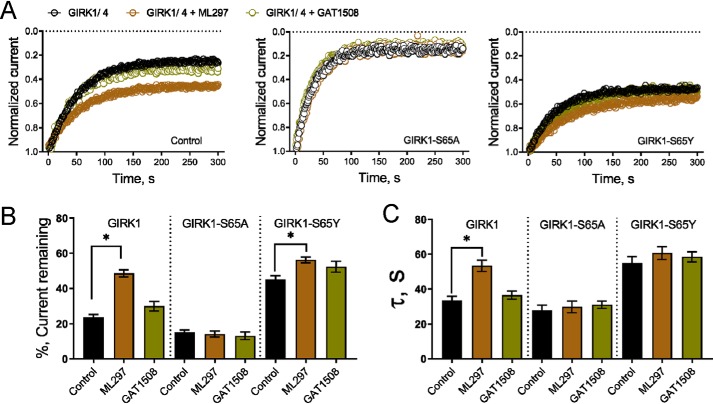
Figure 9.
GIRK1 subunit Slide helix residue Ser-65 is required for the drug-induced enhancement of GIRK activity. Data are currents recorded from HEK293 cells using patch-clamp in the whole-cell mode and are shown as means ± S.E. for 5–6 cells per group. Statistical significance was calculated using unpaired t tests. *, p < 0.01. ML297 and GAT1508 were used at 10 μm. A, left, representative plots of normalized current magnitude against time show that GIRK1/4 channel current decreases in response to light-activated metabolism of PIP2 by the phosphatase 5-ptaseOCRL (control, black open circles). The decrease in current is reduced when GIRK1/4 channels are studied in the presence of ML297 (brown open circle) but not with GAT1508 (olive green open circle). Middle, channels containing GIRK1-S65A subunit currents are more sensitive to 5-ptaseOCRL and are not protected by either compound. Right, in contrast, channels with GIRK1-S65Y subunits are more protected than WT. B, bar graph representing the mean increase in the percentage of GIRK1/4 channel current remaining following activation of 5-ptaseOCRL in the absence (control) or presence of ML297 or GAT1508. C, 5-ptaseOCRL–mediated decrease in GIRK1/4 current is characterized by mono-exponential fits in the presence and absence of the compound indicated. The bar graph shows the mean τ of current inhibition is increased when WT channels are studied with ML297 but not GAT1508. This effect is decreased when GIRK1-S65A subunits are expressed. Channels with GIRK1-S65Y subunits have an increased τ of current inhibition that is not statistically increased with ML297 or GAT1508.