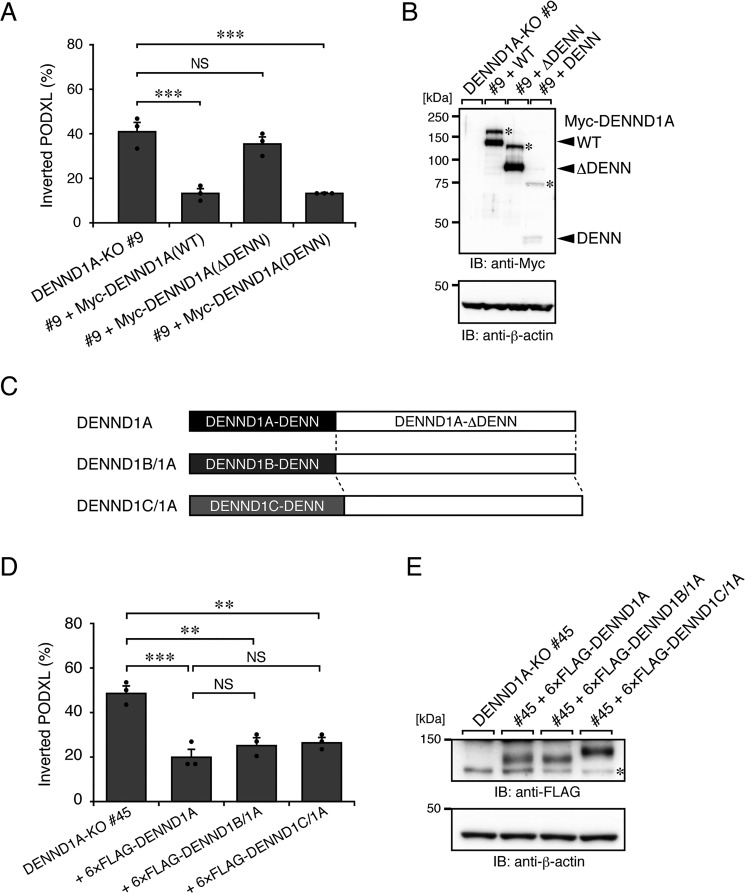
Figure 4.
The N-terminal DENN domain of DENND1A is required for PODXL trafficking in 3D cysts. A, DENND1A-KO (#9) and its rescued cells (+Myc-DENND1A (WT, ΔDENN, or DENN)) were plated on Matrigel and fixed at 42 h after plating, followed by counting of the inverted cysts (30 cysts/condition). The graph shows the means and S.E. (error bars) of three independent experiments. ***, p < 0.001; NS, not significant (Dunnett's test). Representative images are shown in Fig. S3B. B, lysates of the cells used in A were analyzed by immunoblotting (IB) with anti-Myc and anti-β-actin antibodies. The additional higher bands (asterisks) in the blots presumably result from post-translational modifications, such as phosphorylation (30). Alternatively, the DENN domain of DENND1A could form an SDS-insensitive dimer. C, schematic representation of DENND1B/1A and DENND1C/1A chimera proteins. The DENND1B/1A (or DENND1C/1A) chimera protein consists of the DENN domain of human DENND1B (DENND1B-DENN) (or DENND1C (DENND1C-DENN)) fused with the C-terminal part of mouse DENND1A (DENND1A-ΔDENN). D, DENND1A-KO (#45) and its rescued cells (+6×FLAG-tagged DENND1A, DENND1B/1A, or DENND1C/1A) were plated on Matrigel and fixed at 42 h after plating, followed by counting of the inverted PODXL (30 cysts/condition). The graph shows the means and S.E. (error bars) of three independent experiments. ***, p < 0.001; **, p < 0.01; NS, not significant (Tukey's test). Representative images are shown in Fig. S3C. E, lysates of the cells used in D were analyzed by immunoblotting with anti-FLAG and anti-β-actin antibodies. The asterisk shows the predicted 3×FLAG-tagged SpCas9 degradation products.