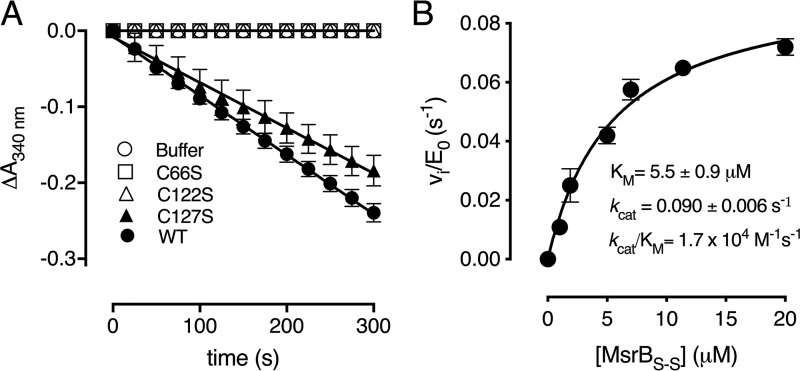
Figure 5.
Thioredoxin only reduces the Cys-66–Cys-122 disulfide. A, progress curves of WT Cd-MsrB and the cysteine mutants (C66S, C122S, and C127S) coupled to the Trx/TrxR pathway, following MetSO reduction, are shown. The C66S and C122S mutants do not consume NADPH, whereas mutating Cys-127 has almost no effect on the reaction rate. B, the reduction of Cys-66–Cys-122 disulfide bond by Trx follows the Michaelis-Menten steady-state kinetics. Increasing concentrations of Met-SO–oxidized Cd-MsrBS-S were used as substrate for thioredoxin. From the Michaelis-Menten curve, the rate of disulfide bond reduction was determined to be 1.7 × 104 m−1 s−1. The data are presented as a mean ± S.D. of three independent technical repeats and the graphs were generated using Prism8.