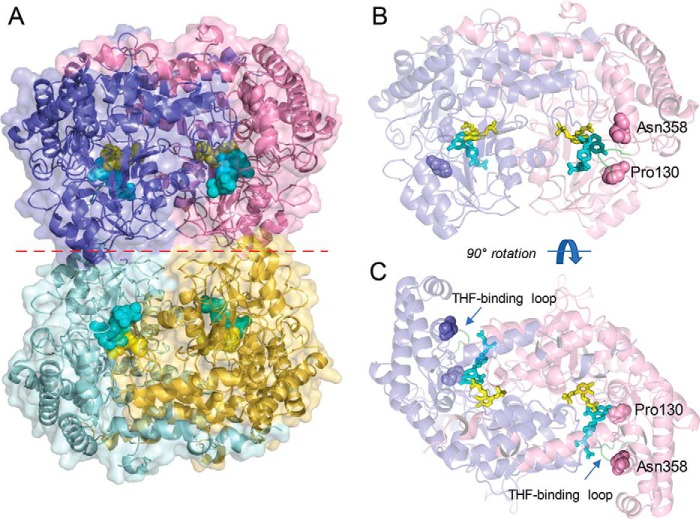
Figure 2.
Overview of the structure of soybean SHMT8. The complex of Essex SHMT8 with PLP-Gly and FTHF is shown. A, structure of the tetramer with the four chains shown in different colors. The two obligate dimers are in purple/pink and cyan/yellow. PLP-Gly and FTHF are shown in the four active sites as spheres in yellow and cyan, respectively. The dimer–dimer interface is indicated by a dashed red line. B, side view of the obligate dimer in the same orientation as in A, showing the protomer–protomer interface, ligands as sticks, and the two residues affected by Forrest polymorphisms. The protein is shown in semitransparent mode to aid in visualization of the ligands. Residues 130 and 358 are shown as spheres. C, top down view of the obligate dimer (90° rotation from B), highlighting how the ligand-binding sites lie at the interface of the two polypeptide chains.