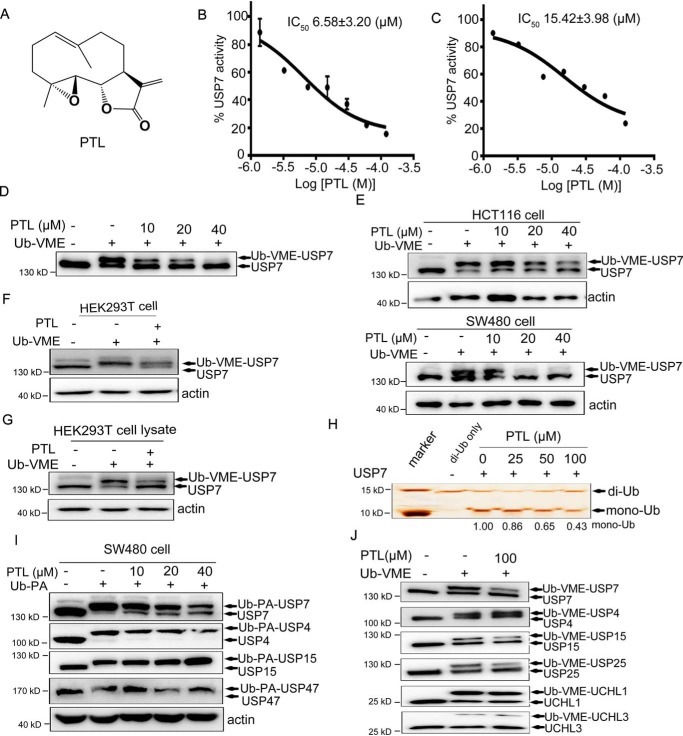
Figure 1.
Identification and biochemical characterization of PTL as a USP7 inhibitor. A, structure of PTL. B, dose-dependent inhibition of USP7 activity by PTL using the Ub-AMC as substrate. The results are presented as mean ± S.D. (n = 2). C, dose-dependent inhibition of USP7 activity by PTL using the Ub-Rho110 as substrate. The results are presented as mean ± S.D. (n = 2). D, purified FLAG-USP7 were treated with PTL, and the Ub-VME probe was added. Samples were subsequently analyzed by Western blotting using anti-USP7 antibody. E, HCT116 and SW480 cells treated with different doses of PTL were collected and lysed, and Ub-VME probes were added into the cell lysates for 30 min. Samples were subsequently analyzed by Western blotting with anti-USP7 antibody. F, HEK293T cells directly incubated with PTL were then collected and labeled with Ub-VME, followed by immunoblot analysis with anti-USP7 antibody. G, HEK293T cell lysates pretreated with or without PTL were labeled with Ub-VME and analyzed by Western blotting. H, recombinant His-USP7 was pretreated with indicated dose of PTL for 40 min and then incubated with K48-linked di-Ub for 3 h. SDS-PAGE and silver staining were employed to analyze the cleavage of di-Ub by USP7 in the presence or absence of PTL. I, SW480 cells were treated with different doses of PTL for 2 h and then labeled with Ub-PA. Individual DUBs were identified using specific antibodies. J, purified FLAG-USP7 and additional deubiquitinating enzymes (FLAG-USP4, FLAG-USP15, FLAG-USP25, FLAG-UCH-L1, and FLAG-UCH-L3) were treated with PTL for 20 min and then labeled with Ub-VME for another 20 min, followed by SDS-PAGE with FLAG-Tag antibody.