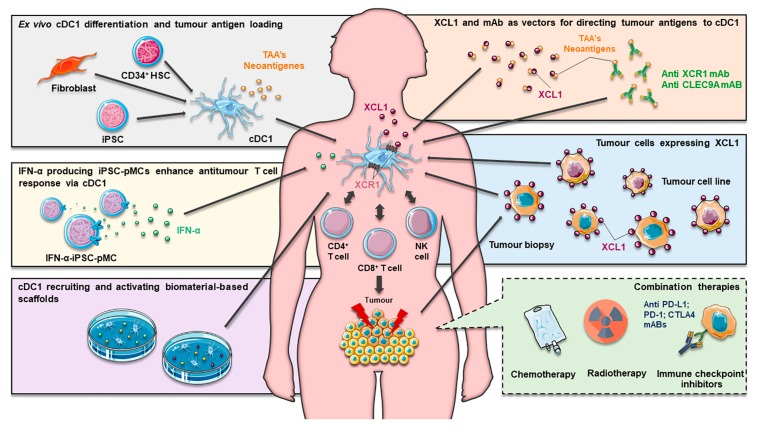
Figure 3.
Summary of current approaches exploring cDC1 in immunotherapy. To overcome the scarcity of natural circulating XCR1+ DCs, which hampers their clinical application, new protocols have allowed their ex vivo generation from CD34+ precursors and iPSCs and their reprograming from fibroblasts. As the resulting cells excel at the cross-presentation and generation of CTL responses, they allow for the formulation of innovative DC-based vaccines, where antigen loading with different TAAs and promising neoantigens potentially generate superior outcomes. Endogenous cDC1 can also be in vivo targeted by using carriers such as XCL1 or monoclonal antibodies to deliver antigens in a specific manner. Likewise, autologous and allogeneic tumor cells can be genetically engineered to express XCL1 and target XCR1+ DCs. Furthermore, IFN-α-iPSC-pMCs can potentiate XCR1+ DC activity by releasing IFN-α in vivo. Additionally, biomaterial-based scaffolds can be designed to release factors that recruit and activate DCs. These strategies aim to boost antigen-specific CD8+ T clone proliferation and responses, as well as enhance CD4+ T and NK cell activity. All these approaches can be potentially combined with conventional cancer therapeutics, namely radiotherapy and chemotherapy, but also with more recently developed immunotherapy strategies, such as immune checkpoint inhibitors. cDC1, Classical dendritic cell 1; CTL, cytotoxic T lymphocyte; DCs, dendritic cells; IFN-α, interferon alpha; IFN-α-iPSC-pMCs, IFN-α producing induced pluripotent stem-cell-derived proliferating myeloid cells; iPSCs, induced pluripotent stem-cells; NK, natural killer; TAAs, tumor-associated antigens; XCL1, X-C Motif Chemokine Ligand 1.