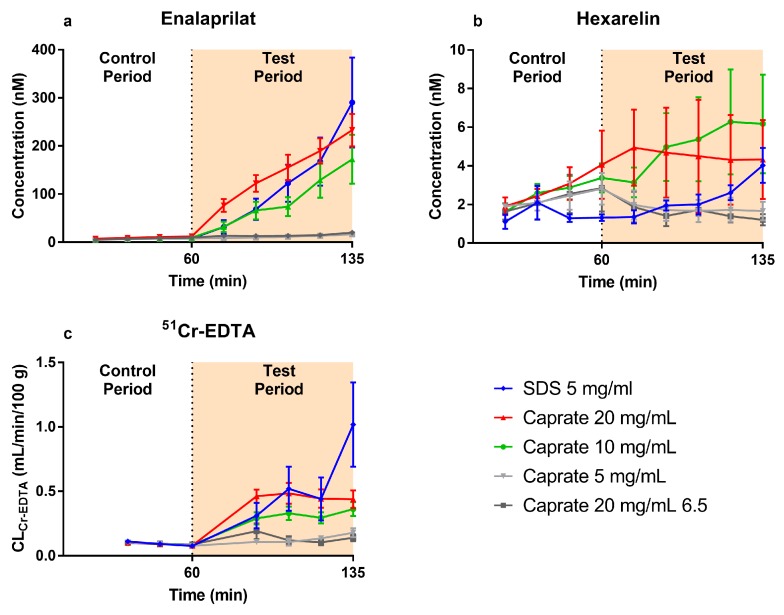
Figure 3.
The mean (±SEM) plasma concentration–time profiles (n = 5/6) of (a) enalaprilat and (b) hexarelin and (c) the blood-to-lumen clearance of 51Cr-EDTA (CLCr-EDTA), following the intestinal perfusions of a control solution for 60 min, followed by a 75-min perfusion of any of five test formulations containing a permeation enhancer: The control solution and all test formulations contained both 100 µM enalaprilat and 90 µM hexarelin. The perfusate pH was 7.4, and the permeation enhancers were sodium dodecyl sulfate (SDS) at 5 mg/mL and caprate at 5, 10, and 20 mg/mL. Caprate at 20 mg/mL was also tested at pH 6.5, where its solubility was 2 mg/mL instead of 5 mg/mL at pH 7.4.