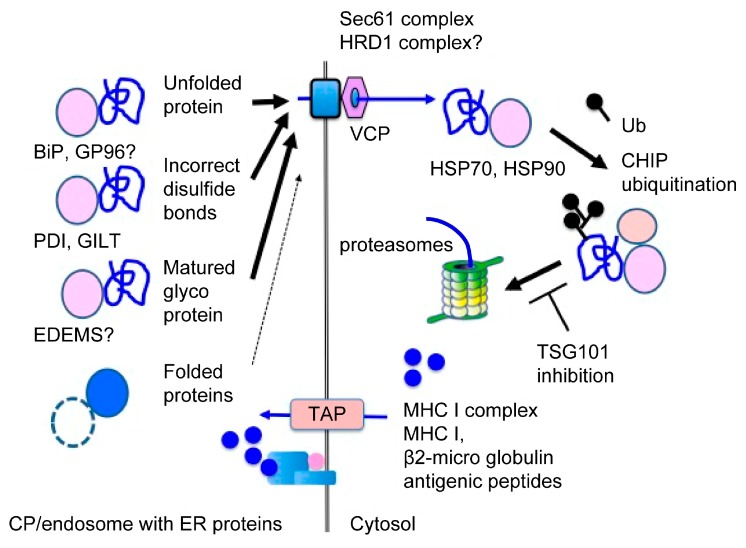
Figure 2.
Recognition of extracellular proteins in CP. In ERAD-dependent processing, extracellular proteins are unfolded by ROS, resulting in nonspecific disulfide bonds in endocytic compartments. These unfolded extracellular proteins lose their activity and are preferentially recognized as ERAD substrates by ER-resident molecular chaperones, such as BiP, PDI, or ER-degradation enhancing α-mannosidase-like proteins (EDEMs). After recognition, these proteins are retro-transported into the cytosol. In contrast, self-proteins, which are properly folded in these cellular compartments, are not recognized as ERAD substrates and remain in endocytic compartments. After retro-transportation into the cytosol, extracellular proteins are recognized by cytosolic molecular chaperones, such as Hsp70 or Hsp90. After recognition, extracellular proteins are processed by the ubiquitin-proteasome system (UPS) into antigenic peptides and transported into membranous compartments in a transporter associated with antigen processing (TAP)-dependent manner. These two recognition steps indicate that unfolding and recognition of extracellular proteins are critical for CP.