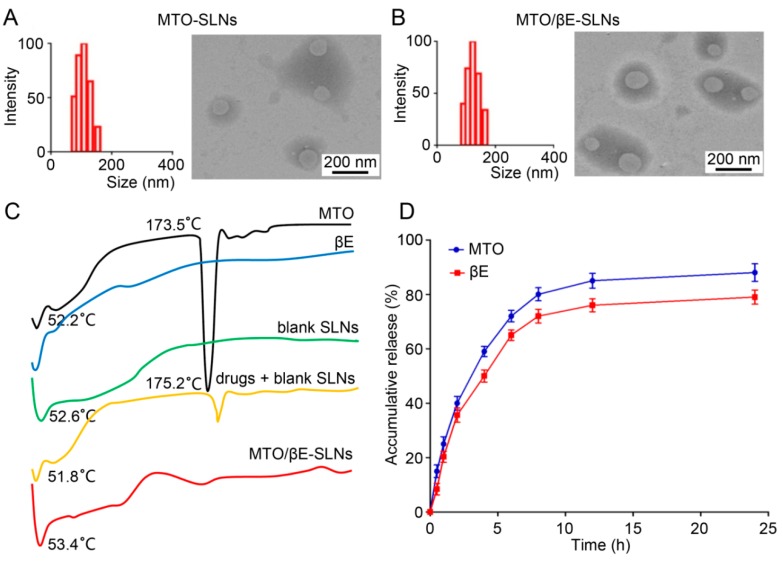
Figure 1.
(A,B) The particle size and TEM images of (A) MTO-SLNs and (B) MTO/βE-SLNs. (C) The differential scanning calorimetry (DSC) analysis of MTO powder, βE, blank SLN powder, physical mixture of drugs (MTO/βE) and blank SLNs powder, MTO/βE-SLNs powder. (D) The in vitro release of MTO and βE from MTO/βE-SLNs at pH 7.4. Data represent means ± SD (n = 3). ANOVA performed for the release profile of MTO and βE after 24 h reveals a statistically reliable difference at 95% confidence. The accumulated release of MTO was observed to be higher than that of βE after 24 h. Both MTO and βE exhibited a similar release tendency of the drug in this study.