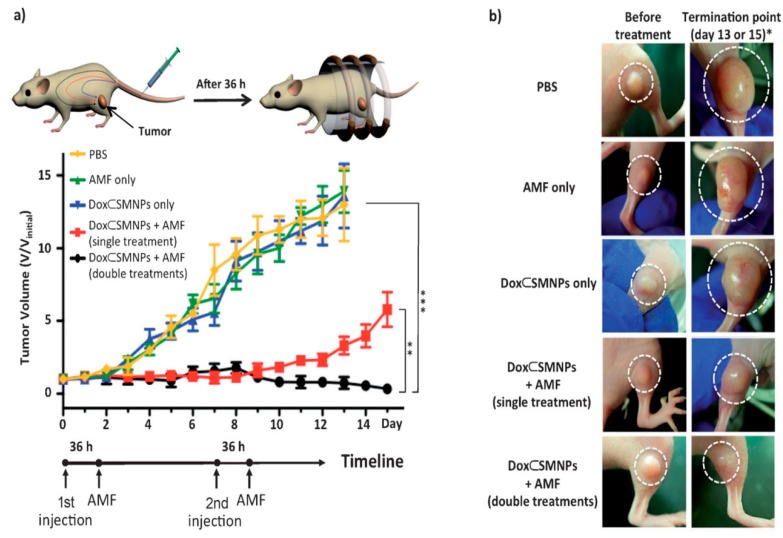
Figure 1.
Evaluation of in vivo therapeutic efficacy. (a) Treatment scheme of DoxSMNPs in mouse and results of the tumor volume change over the course of the treatment (15 days) in DLD-1 xenografted mice (n = 3) treated with DoxSMNPs (w/and w/o application of AMF) and other controls (AMF only and PBS only). All injections were done on day 0 (and day 7 for the double injection group) when the tumor volume reached 100 mm3; AMF application was performed at 36 h post-injection. The best tumor suppression result was observed in the group treated with a double injection of DoxSMNPs with AMF application. The group treated with a single injection of DoxSMNPs with AMF and the other control groups (i.e., treated with DoxSMNPs only, AMF only and PBS) show either a smaller degree or none of tumor suppression effects (** p ≤ 0.01; *** p ≤ 0.001). (b) Tumor images of groups treated with DoxSMNPs w/and w/o application of AMF and other controls, before treatment (left panels) and at the termination point (right panels). * The termination point of the experiment occurred either on day 15 or when the tumor volume reached 1500 mm3. The figure was reproduced from [59] after permission from John Wiley and Sons.