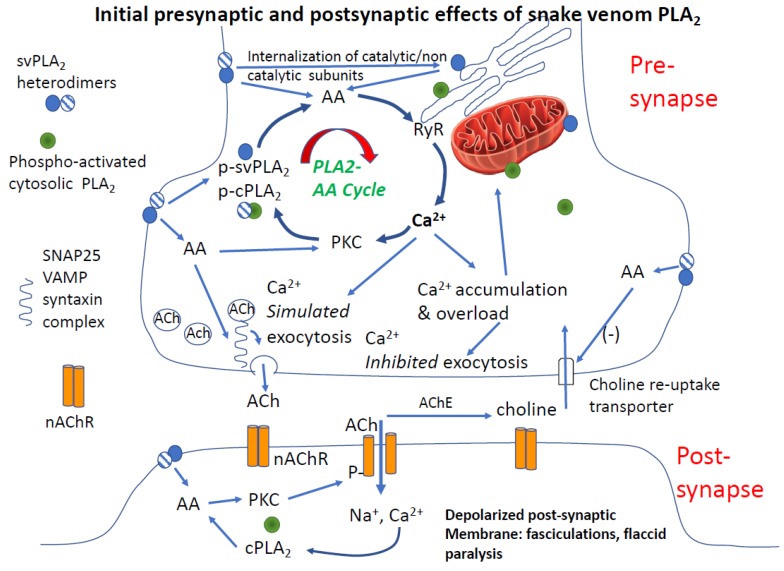
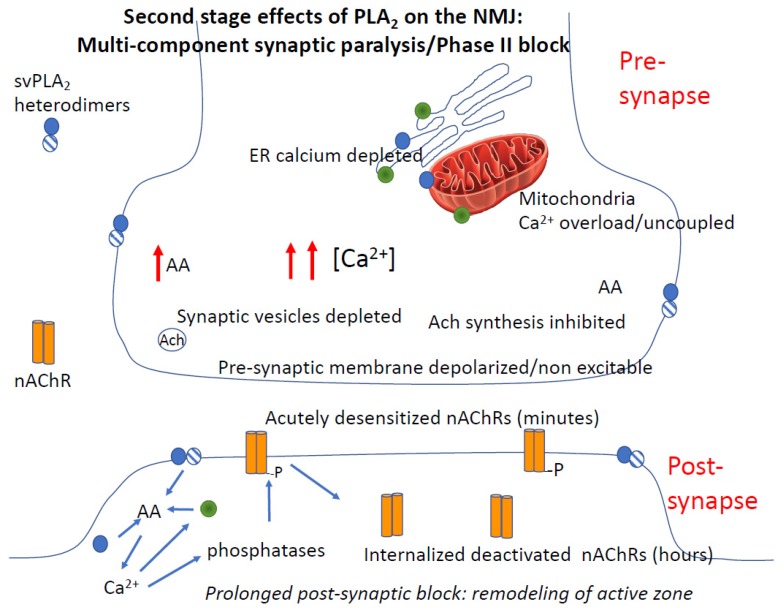
Figure 3.
Multi-site failure of synaptic transmission mediated by svPLA2s. Upper panel shows cycle of amplification of arachidonic acid and calcium signaling causing rapid depletion of pre-synaptic acetylcholine vesicles, increases in intracellular Ca2+ [Ca2+]i and acute desensitization of post-synaptic nicotinic acetylcholine receptors. Key events include snake venom (svPLA2)-mediated increase in pre-synaptic arachidonic acid (AA), and increases in pre-synaptic [Ca2+]i from release from intra-neuronal stores in the endoplasmic reticulum and augmented by voltage-gated calcium channels (not depicted). These actions are amplified by direct AA activation of protein kinase C, which facilitates activation of the vesicle fusion protein complex. Both catalytic and non-catalytic PLA2 subunits (shaded and cross-hatched circles, respectively) are potentially able to co-activate endogenous PLA2. Activation of intracellular, endogenous, PLA2 is part of the amplification cycle. The net effect is depletion of pre-synaptic transmitter vesicles and mitochondrial Ca2+ uptake. AA inhibition of the choline re-uptake transporter amplifies the decrease of releasable acetylcholine. Lower panel depicts the short and longer-term effects of PLA2 at the neuromuscular junction. Following the initial burst of acetylcholine release, post-junctional acetylcholine receptors are desensitized and then inactivated (dephosphorylated, internalized) analogous to their state in a phase II neuromuscular block produced by large/repeated doses of succinylcholine. As in the pre-synapse, PLA2 mediates a self-amplifying cycle of increase in arachidonic acid, intracellular calcium, and calcium-sensitive phosphatase activation. The process is augmented both by internalization of svPLA2 and/or activation of endogenous PLA2. The post-synaptic membrane is now depolarized and unexcitable for a prolonged period.