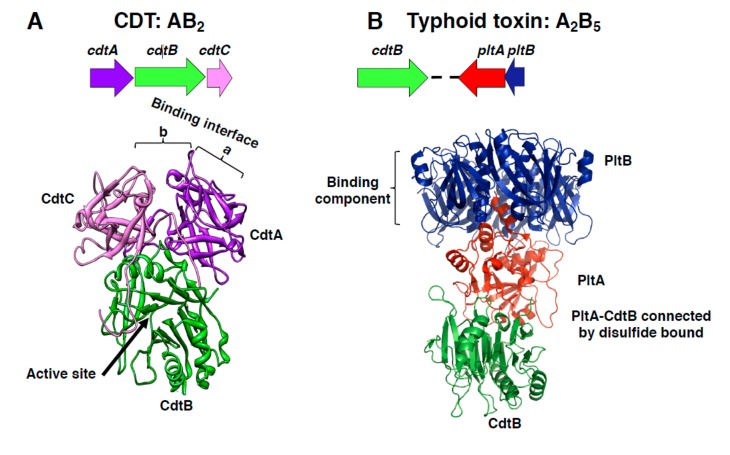
Figure 1.
Cytolethal distending toxin (CDT) and typhoid toxin structure. (A) Schematic representation of the CDT operon from H. ducreyi and the crystal structure of the holotoxin, adapted from Nesic et al. [7], PDB access number: 1SR4. The CdtB is the active subunit, while the CdtA and CdtC accessory subunits contribute to the binding interface, composed by the aromatic patch in CdtA (a) and an adjacent deep groove (b). (B) Schematic representation of the Salmonella enterica serovar Typhi islet encoding for the typhoid toxin genes and crystal structure of the holotoxin, adapted from Song et al., PDB access number: 4K6L [10]. This toxin contains two active subunits: CdtB, homologous to mammalian DNase I, a characteristic shared with CDTs, and the ADP ribosyl transferase PltA, and they are linked to each other by a disulfide bound. The binding moiety is formed by a pentameric disc made by five PltB monomers.