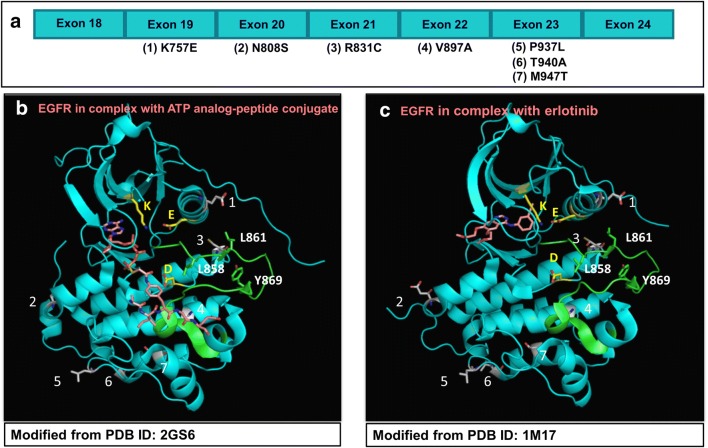
Fig. 1.
Structures of HCC-derived EGFR mutants, based on wild type EGFRs [25, 26]. Seven HCC-derived EGFR mutants arranged according to their codon position in EGFR exon 18–24, shown in graphic (a). Structures of these HCC-derived EGFR mutant residues in the active EGFR kinase domains are represented by modification of the wild type EGFR residues (PDB ID: 2GS6, and PDB ID: 1M17) [25, 26], with PyMOL Molecular Graphic System (Version 2.3.2) (Schrodinger, New York, NY, USA) (b, c, respectively). Overall, EGFR presented in cartoon with cyan color, whereas its conserved residues with ionic charges presented in sticks with carbon atom in yellow (K, K745; E, E762; D, D837), activation loop (855–884) in green and its important residues in sticks with carbon atom in green (L858, L861, Y869). HCC-derived EGFR mutant residues in this study presented in sticks with carbon atom in grey-white, numbering of the mutant residues as follows: 1, K757E; 2, N808S; 3, R831C; 4, V897A; 5, P937L; 6, T940A; 7, M947T. ATP analog-peptide conjugate and erlotinib presented in sticks with carbon atom in rose. In all sticks, nitrogen atom presented in blue, oxygen atom in red, and phosphorus atom in orange