Abstract
Background
Patients with rheumatoid arthritis (RA) who develop interstitial lung disease (RA-ILD), show features of usual interstitial pneumonia (UIP) on high-resolution computed tomography (HRCT). This retrospective exploratory clinical study aimed to investigate the association between mutations in the MUC5B gene and clinical outcome in patients with RA, with or without RA-ILD, using whole-exome sequencing (WES).
Material/Methods
WES was performed using peripheral blood samples for mutations in the MUC5B gene in 51 patients diagnosed with RA without ILD, and 45 patients with RA-ILD. The cumulative incidence in acute exacerbations of RA-ILD and variables associated with acute exacerbations of RA-ILD were analyzed.
Results
In patients with RA-ILD, the main genetic variants of MUC5B were identified, with an odds ratio (OR) of 3.410 (p=0.013). Nine patients with RA without ILD (17.6%) and 19 patients with RA-ILD (42.2%) expressed MUC5B variants. Patients with RA-ILD carrying MUC5B variants had a significantly increased duration of RA-ILD (p=0.03) and showed a UIP pattern on lung HRCT (p=0.01). Acute exacerbations of RA-ILD occurred in 25 patients during follow-up, including 13 patients with mutant MUC5B and 12 patients with wildtype MUC5B. Univariate analysis showed that MUC5B mutations (p=0.043), older age of onset of RA (p=0.041), increased serum anti-citrullinated protein antibodies (ACPAs) (p=0.033), and a UIP imaging pattern on HRCT (p=0.015) were significantly correlated with acute exacerbations of RA-ILD. However, these findings were not supported by multivariate analysis (p=0.065).
Conclusions
The carrier status of MUC5B variants was an indicator of reduced prognosis and increased exacerbations of RA-ILD.
MeSH Keywords: Arthritis, Juvenile; Lung Diseases, Interstitial; Mucin-5B
Background
Rheumatoid arthritis (RA) is an autoimmune disease with clinical features that include symmetrical arthritis and synovitis. Extra-articular complications occur in approximately 50% of patients with RA, and clinical or subclinical pulmonary involvement is an extra-articular feature of the RA phenotype [1]. Interstitial lung disease (ILD) is the main manifestation of lung involvement and results in a three-fold increase in mortality in patients with RA-ILD when compared with patients with RA without ILD [2]. Patients who develop RA-ILD show features of usual interstitial pneumonia (UIP) on high-resolution computed tomography (HRCT). Also, patients with collagen vascular disease-associated ILD who have acute deterioration in respiratory status, including increased dyspnea, new bilateral ground-glass opacities, or consolidation on CT imaging, in the absence of infection, pulmonary embolism, or lung injury have an in-hospital mortality rate comparable with patients with idiopathic pulmonary fibrosis (IPF) [3,4]. However, the pathogenesis and clinical outcome of acute exacerbations in patients with RA-ILD remain poorly understood.
Given the similarities between IPF and RA-ILD, including UIP seen on HRCT imaging [5], the association with cigarette smoking [6], and male gender [7], the conditions may share molecular risk factors. Juge et al. [8] reported increased TERT, RTEL1, PARN, and SFPTC gene mutations in patients with RA-ILD when compared with 1,010 control individuals of European ancestry, with an odds ratio (OR) of 3.17 and a 95% confidence interval (CI) of 1.53–6.12 (p<0.001). In a further study [9], these investigators also showed that the MUC5B promoter variant rs35705950 was associated with UIP on imaging in patients with RA-ILD (OR, 6.1; 95% CI, 2.9–3.1; p<0.001). Because MUC5B encodes respiratory tract mucin 5B glycoprotein, which has a role in mucociliary clearance, these investigators proposed that genetically-driven overexpression of MUC5B may result in high levels of the protein, which would impair mucociliary clearance and disrupt normal lung repair mechanisms [10]. However, Peljto et al. [11] reported that MUC5B promoter polymorphisms were associated with improved survival in patients with IPF (p<0.001). Older age at onset of ILD (HR 1.11; p=0.01), a UIP pattern on HRCT (HR 1.95; p=0.03), and treatment with methotrexate (HR 3.04; p=0.001) have all been associated with exacerbation of acute RA-ILD, which is a prognostic indicator of poor clinical outcome (HR 2.47; p=0.003) [12]. However, no genotype and phenotype correlation analysis has been performed in RA-ILD in a Chinese population.
Therefore, this retrospective exploratory clinical study aimed to investigate the association between mutations in the MUC5B gene and clinical outcome in patients with RA, with or without RA-ILD, using whole-exome sequencing (WES).
Material and Methods
Patients studied
This study was conducted according to the principles of the Declaration of Helsinki [13] and was approved by the Ethics Committee of Peking Union Medical College Hospital (Approval No. JS1127). A total of 96 outpatients admitted to Peking Union Medical College Hospital between 2016 and 2019 were enrolled in the study, including 51 patients with rheumatoid arthritis (RA) without interstitial lung disease (ILD) and 45 patients with RA and interstitial lung disease (RA-ILD). The diagnosis of RA was confirmed using details from the medical records, according to the diagnostic criteria of the 2010 European League against Rheumatism and American College of Rheumatology (ACR/EULAR) criteria [14]. All patients provided written informed consent to participate in the study.
The diagnosis of ILD was based on the clinical presentation, pulmonary function tests, and high-resolution computed tomography (HRCT) imaging findings of the chest. Patients with chest HRCT findings that included bilateral ground-glass opacities, reticular opacities, and reticular (honeycomb) fibrosis were reviewed by radiologists and pulmonologists, who confirmed the diagnosis of ILD. The HRCT imaging patterns were identified as usual interstitial pneumonia (UIP), possible UIP, or inconsistent with UIP, based on current international clinical criteria [15]. Patients with lung disease associated with occupational or environmental exposure, drug use, or other known causes of ILD were excluded from the study. Patients underwent six-monthly follow-up after diagnosis to monitor acute exacerbations. The clinical data collected included the results of routine blood tests, serology, imaging, and pulmonary function testing.
Main clinical outcomes
The primary clinical outcomes or endpoints included acute exacerbations of RA-ILD and all-cause mortality. Acute exacerbations were defined according to five criteria: previously diagnosed RA-ILD; idiopathic worsening of dyspnea within one month of onset of RA-ILD; new bilateral ground-glass lung opacities or consolidation with reticular (honeycomb) fibrosis on HRCT, no evidence of lung infection or a negative sputum culture test; and exclusion of known causes of respiratory dysfunction, such as left heart failure, pulmonary embolism, or lung injury.
Whole-exome sequencing (WES)
Peripheral blood samples were extracted from patients, following informed consent. Each patient provided 1 ml of peripheral blood. All the samples were stored at −80°C for further analysis. WES was performed on the peripheral blood samples following DNA extraction and preparation of the DNA library [16]. Sequencing, read mapping to the reference sequence, and data processing were performed according the methods previously described [16].
Statistical analysis
Data were analyzed using SPSS version 23.0 software (IBM Statistics, Chicago, IL, USA). Pearson’s chi-squared (χ2) test, Fisher’s exact test, and the Kruskal-Wallis test were performed to analyze the relationships between different variables. Kaplan-Meier survival analysis and the Cox proportional hazard model were performed to evaluate the relationship between MUC5B gene mutation status, and the primary endpoints of acute exacerbation or all-cause mortality in patients with RA with and without ILD. All data were presented as the mean±standard deviation (SD). A p-value <0.05 was considered to be statistically significant.
Results
Clinicopathological findings of the patients with rheumatoid arthritis (RA) with and without interstitial lung disease (ILD)
The clinicopathological characteristics of patients with RA, with and without ILD, are shown in Table 1. A total of 51 patients with RA without ILD and 45 patients with RA-ILD were enrolled in the study. The demographic and clinical features of the two groups were compared, including age, gender, and laboratory findings. Several clinicopathological features showed significant difference between the two groups, including age at inclusion into the study (p<0.001), age at onset of RA (p<0.005), serum levels of anti-citrullinated protein antibodies (ACPAs) (p=0.01), the erythrocyte sedimentation rate (ESR) (p=0.03), prednisone treatment (p<0.001), methotrexate treatment (p<0.001), and cyclophosphamide treatment (p=0.008).
Table 1.
Demographic and phenotypic spectrum of patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and RA without ILD.
| Characteristics | All (N=96) | Patients with RA (N=51) | Patients with RA-ILD (N=45) | p-Value |
|---|---|---|---|---|
| Male gender. No./Total No. (%) | 32/96 (33.3) | 14/51 (27.4) | 18/45 (40.0) | 0.203 |
| Age at inclusion (years) | 55.5±11.6 | 46.1±12.2 | 59.5±8.7 | <0.001* |
| Age at onset of RA (years) | 47.5±13.2 | 40.4±11.7 | 54.0±12.2 | 0.005* |
| Duration of RA (years) | 6.8±5.6 | 6.0±4.2 | 7.2±6.2 | 0.549 |
| Age at onset of ILD (years) | 56.1±7.8 | |||
| Duration of ILD (years) | 2.2±1.7 | |||
| Ever smoker. No./Total No. (%) | 16/96 (16.7) | 7/51 (13.7) | 9/45 (20.0) | 0.427 |
| Manifestation of RA | ||||
| Erosive disease. No./Total No. (%) | 75/96 (78.1) | 44/51 (86.4) | 31/45 (68.9) | 0.051 |
| Laboratory indicators | ||||
| ACPA titer | 900.9±1042.8 | 423.4±697.0 | 1139.6±1112.8 | 0.012* |
| Rheumatoid factor titer | 294.8±659.0 | 112.4±121.6 | 372.2±773.0 | 0.220 |
| ESR | 31.5±24.4 | 22.2±15.5 | 36.0±26.7 | 0.033* |
| Pattern of HRCT | ||||
| NSIP or possible NSIP. No./Total No. (%) | 6/45 (13.3) | |||
| UIP or possible UIP. No./Total No. (%) | 18/45 (40.0) | |||
| Pulmonary function | ||||
| Forced vital capacity (% of predicted value) | 79.9±20.1 | |||
| DLCO (% of predicted value) | 58.4±13.4 | |||
| Total lung capacity (% of predicted value) | 79.4±18.6 | |||
| Treatments | ||||
| Pred | 45/96 (46.9) | 14/51 (27.4) | 31/45 (68.9) | 0.001* |
| MTX | 31/96 (32.3) | 628/51 (54.9) | 3/45 (6.7) | <0.001* |
| CTX | 11/96 (11.5) | 0/51 (0) | 11/45 (24.4) | 0.008* |
RA – rheumatoid arthritis; ILD – interstitial lung disease; ACPA – anti-citrullinated protein antibody; ESR – erythrocyte sedimentation rate; NSIP – non-specific interstitial pneumonia; UIP – usual interstitial pneumonia; DLCO – diffusion capacity for carbon monoxide; PRED – prednisone; MTX – methotrexate; CTX – cyclophosphamide; HRCT – high-resolution computed tomography.
Older patients with RA were more commonly affected by ILD than younger patients (54.0±12.2 vs. 40.4±11.7 years; p=0.005). Patients with RA-ILD had showed a significantly higher mean level of inflammatory indicators and serum antibodies than patients with RA without ILD, including the erythrocyte sedimentation rate (ESR) (36.0±26.7 vs. 22.2±15.5 mm/h; p=0.033) and ACPA (1139.6±112.8 vs. 423.4±697.0 U/mL; p=0.012). These findings supported previous studies that showed the production of ACPA in the lungs [17], and also that patients with serum anti-citrullinated protein antibodies (ACPAs) have a genetic predisposition to developing pulmonary fibrosis via targeting of citrullinated epitopes [18]. Also, methotrexate treatment has been reported as a prognostic indicator in RA-ILD [19], and in this study, patients with RA-ILD rarely received treatment with methotrexate (54.9% vs. 6.7%, p<0.001).
MUC5B mutations
The common genes associated with cell senescence and telomere shortening or surfactant secretion in ILD, including TERT, TERC, DKC1, RTEL1, PARN, SFTPC, ABCA3, and SFTPA2 were studied in all patients with RA to investigate the mechanisms involved in RA-ILD. There were 434 and 345 single nucleotide polymorphisms (SNPs) detected in patients with RA with and without ILD, and 26 rare SNPs, with a minor allele frequency (MAF) <0.01 that were detected, including missense or splicing variants, nonsynonymous variants, and deleterious variants. These findings indicated that the MUC5B gene mutation alone might be a significant predictor of RA-ILD compared with RA without ILD (OR 3.410, 95% CI 1.343–8.659; p=0.013). No significant difference was observed between other potential risk indicators for ILD in patients with RA with and without ILD (Figure 1).
Figure 1.
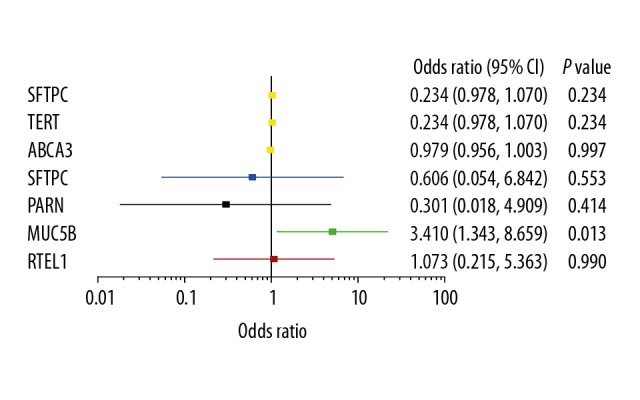
The association between the interstitial pulmonary fibrosis (IPF) panel mutations in patients with rheumatoid arthritis (RA) without interstitial lung disease (ILD) and patients with RA-ILD. The forest plots show the odds ratio (OR) and 95% confidence interval (CI). The boxes in the plot indicate the odds ratio (OR), and the horizontal lines represent the 95% CI. The vertical line indicates a mean OR=1. The results show a lack of significance for the IPF panel between patients with RA without ILD and patients with RA-ILD, except for MUC5B mutation status.
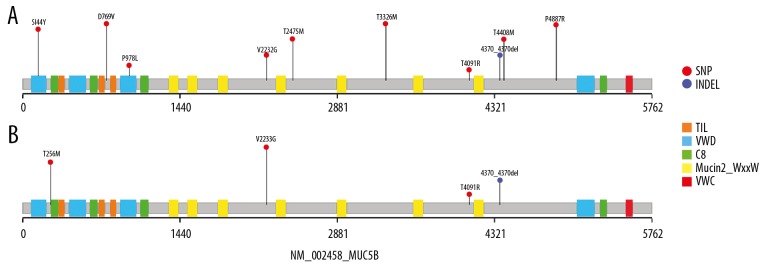
Based on the above findings, further studies focused on the MUC5B gene variants. There were 48 and 32 variants identified in patients with RA with and without ILD, including exon, splicing, and nonsynonymous variants. Because the rare variants included few functional exchanges, we mainly focused on the rare (MAF <0.01) and deleterious variants, which were filtered using the Exome Aggregation Consortium (ExAC) database, the SIFT database, the Polymorphism Phenotyping (PolyPhen) tool, the MutationTaster web-based application, and the Combined Annotation Dependent Depletion (CADD) tool. There were 19 patients with 10 variants that included 15 cases of missense mutation (78.9%) and four cases of non-frameshift deletion mutation (21.1%) that were detected in the 45 patients with RA-ILD, representing an overall mutation rate of 42.2% (19/45). The MUC5B mutant domains in patients with RA-ILD are summarized in Figure 2A. The majority of variants (80%) were located in the O-glycosylation areas, and two were located in the von Willebrand factor type D domain (vWD). The overall MUC5B mutation rate in patients with RA was 17.6% (9/51), with seven cases of missense mutation and two cases of non-frameshift deletion mutation. The mutant domains of MUC5B detected in patients with RA are shown in Figure 2B, and all four variants were found in the O-glycosylation area.
Figure 2.
Gene linkage diagram of the MUC5B gene and the location of the rare variants. Single nucleotide polymorphisms (SNPs) are indicated by amino acid change and position. All the SNPs were confirmed by a minor allele frequency of 0.01 or less. There were 10 variants identified in patients with rheumatoid arthritis and interstitial lung disease (RA-ILD) (A), and four variants were found in patients with RA without ILD (B). Of these variants, eight were restricted to patients with RA-ILD. The red circle indicates the mutant type of SNP. The blue circle indicates the mutant type of insertion-deletion mutation (indel). The different rectangles show the different domains of MUC5B. Orange indicates the trypsin inhibitor-like cysteine-rich (TIL) domain; blue indicates the von Willebrand factor type D domain (vWD); green indicates the C8 domain; yellow indicates the Mucin-2 protein WxxW (PF13330) domain; and red indicates the von Willebrand factor type C (VWC) domain.
The association between MUC5B mutations and clinical features
Patients were divided into the mutant-type (MT) group and the wild-type (WT) group, according to the mutation status of MUC5B for further comparison of the patient clinicopathological characteristics. The associations between MUC5B mutations and the clinical features of the study participants are summarized in Table 2. Patients with RA-ILD with MUC5B mutations had a significantly longer duration of ILD than patients with RA-ILD without MUC5B mutations (3.0±1.6 years vs. 1.9±1.4 years; p=0.03). Also, the UIP pattern of lung disease was found in 63.2% (12/19) of patients with MUC5B mutations, but only 26.9% (7/26) of patients with wild-type MUC5B (p=0.01). Age at onset of ILD (p=0.439), RA duration (p=0.537), laboratory indicators, or pulmonary function showed no significant difference between individuals with and without MUC5B gene mutations.
Table 2.
Demographic and phenotypic characteristics of patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and RA without ILD according to MUC5B gene mutation status.
| RA-ILD group | RA group | |||||
|---|---|---|---|---|---|---|
| MT MUC5B (N=19) | WT MUC5B (N=26) | p-Value | MT MUC5B (N=9) | WT MUC5B (N=42) | p-Value | |
| Female gender. No./Total No. (%) | 12/19 (63.2) | 15/26 (57.7) | 0.77 | 4/9 (44.4) | 26/42 (61.9) | 0.46 |
| Age at inclusion (years) | 62.1±8.5 | 57.7±8.6 | 0.11 | 40.5±6.4 | 49.2±13.4 | 0.38 |
| Age at RA onset (years) | 50.8±11.9 | 52.5±12.5 | 0.74 | 37.0±5.7 | 41.4±12.3 | 0.63 |
| RA duration (years) | 7.1±5.1 | 7.1±7.0 | 0.98 | 3.5±0.7 | 4.6±4.4 | 0.37 |
| Age at onset of ILD (years) | 57.6±6.9 | 55.2±8.4 | 0.40 | |||
| ILD duration (years) | 3.0±1.6 | 1.9±1.4 | 0.03* | |||
| Ever smoker. No./Total No. (%) | 3/19 (15.8) | 5/26 (19.2) | 0.99 | 2/9 (22.2) | 14/42 (38.1) | 0.70 |
| Erosive disease. No./Total No. (%) | 12/19 (63.2) | 19/2 (73.1) | 0.53 | 4/9 (44.4) | 31/42 (73.8) | 0.12 |
| Laboratory indicators | ||||||
| ACPA titer | 944.8±1158.0 | 1321.8±1105.8 | 0.38 | 1700.0±2121.3 | 560.9±729.8 | 0.58 |
| Rheumatoid factor titer | 145.2±114.3 | 568.9±1011.7 | 0.13 | 121.4±58.5 | 89.8±114.8 | 0.71 |
| ESR | 29.8±15.2 | 40.6±32.3 | 0.24 | 28.4±19.1 | 22.9±16.6 | 0.68 |
| Pattern on HRCT | ||||||
| NSIP or possible NSIP. No./Total No. (%) | 2/19 (10.5) | 4/26 (15.4) | 0.99 | |||
| UIP or possible UIP. No./Total No. (%) | 12/19 (63.2) | 7/26 (26.9) | 0.01* | |||
| Pulmonary function | ||||||
| Forced vital capacity (% of predicted value) | 78.5±17.4 | 80.5±22.8 | 0.81 | |||
| DLCO (% of predicted value) | 59.2±13.2 | 58.2±14.2 | 0.86 | |||
| Total lung capacity (% of predicted value) | 80.8±21.5 | 78.6±17.8 | 0.79 | |||
| Acute exacerbation. No./Total No. (%) | 13/19 (68.4) | 12/26 (46.2) | 0.22 | |||
MT – mutant-type; WT – wild-type; ILD – interstitial lung disease; ACPA – anti-citrullinated protein antibody; ESR – erythrocyte sedimentation rate; NSIP – non-specific interstitial pneumonia; UIP – usual interstitial pneumonia; DLCO – diffusion capacity for carbon monoxide; HRCT – high-resolution computed tomography.
MUC5B gene mutations and clinical outcome
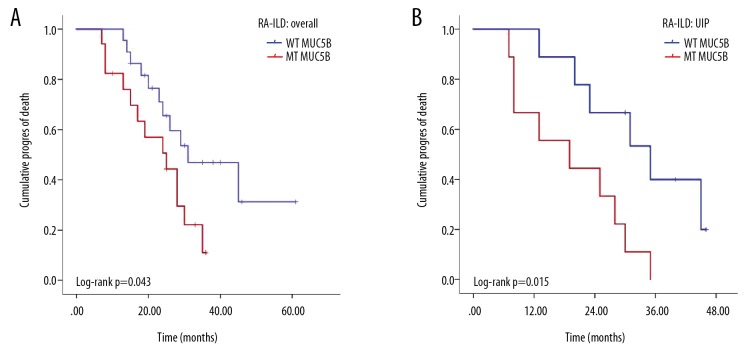
The primary outcome of acute exacerbation of ILD occurred in 25 patients (55.6%) during a median follow-up time of 25.0 months (interquartile range, 7–61 months). Four patients died during follow-up (8.9%), two patients had MUC5B mutations, and they died due from severe infection and heart failure. Of the other two patients with wild-type MUC5B, one patient died from advanced lung cancer, and the other died from severe infection. In the RA-ILD cohort, patients carrying MUC5B variants had a higher risk of acute exacerbation or death (HR, 2.308, 95% CI 1.006–5.292; log-rank p=0.0430 (Figure 3A). In patients with a UIP lung imaging pattern on high-resolution computed tomography (HRCT), MUC5B gene mutations were associated with a significantly shorter interval to acute exacerbation or death compared with patients with the wild-type gene (19.2±3.6 vs. 33.2±3.9; HR, 4.115, 95% CI 1.196–14.164; log-rank p=0.015) (Figure 3B). Older age at onset of RA (HR, 2.916; p=0.041) and higher ACPAs titers (HR, 3.949; p=0.033) were associated with acute exacerbation or death.
Figure 3.
Kaplan-Meier survival curves based on the MUC5B gene mutation status in patients in the rheumatoid arthritis with interstitial lung disease (RA-ILD) cohort. (A) All the patients in the RA-ILD cohort. (B) The usual interstitial pneumonia (UIP) pattern in patients with RA-ILD.
Multivariate analysis was used to study the associations between MUC5B mutations and clinical outcomes further. MUC5B mutation status (HR 2.312, 95% CI 0.951–5.620; p=0.065) was not an independent predictive factor of outcome in patients with RA-ILD, and age at RA onset and UIP were not associated with an acute clinical exacerbation or all-cause mortality (Table 3).
Table 3.
Univariate and multivariate Cox proportional hazards analysis of risk factors for acute exacerbation or all-cause mortality in the rheumatoid arthritis-associated interstitial lung disease (RA-ILD) cohort.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Gender | ||||
| Female | 1 | 0.832 | 1 | 0.539 |
| Male | 1.089 (0.485–2.445) | 0.507 (0.058–4.417) | ||
| Age at inclusion (years) | ||||
| ≤50 | 1 | 0.269 | 1 | 0.432 |
| >50 | 1.978 (0.589–6.639) | 1.349 (0.483–5.420) | ||
| Age at onset of RA (years) | ||||
| ≤50 | 1 | 0.041 | 1 | 0.171 |
| >50 | 2.916 (1.037–8.202) | 5.247 (0.489–56.363) | ||
| RA duration (years) | ||||
| ≤10 | 1 | 0.06 | 1 | 0.882 |
| >10 | 0.245 (0.054–1.101) | 1.158 (0.166–8.073) | ||
| Ever smoker | ||||
| Yes | 1 | 0.39 | 1 | 0.409 |
| No | 0.39 (0.572–4.075) | 0.627 (0.207–1.899) | ||
| ACPA titer | ||||
| ≤500 | 1 | 0.033 | 1 | 0.528 |
| >500 | 3.949 (1.119–13.932) | 0.722 (0.262–1.989) | ||
| UIP pattern on HRCT | ||||
| No | 1 | 0.015 | 1 | 0.297 |
| Yes | 4.115 (1.196–14.164) | 1.693 (0.630–4.551) | ||
| DLCO (% of predicted value) | ||||
| >55 | 1 | 0.620 | 1 | 0.830 |
| ≤55 | 0.783 (0.295–2.081) | 1.101 (0.458–2.644) | ||
| MUC5B mutation | ||||
| WT | 1 | 0.043 | 1 | 0.065 |
| MT | 2.308 (1.006–5.292) | 2.312 (0.951–5.620) | ||
HR – hazard ratio; CI – confidence interval; WT – wild-type; MT – mutant-type; RA – rheumatoid arthritis; ILD – interstitial lung disease; ACPA – anti-citrullinated protein antibody; NSIP – non-specific interstitial pneumonia; UIP – usual interstitial pneumonia; DLCO – diffusion capacity for carbon monoxide; HRCT – high-resolution computed tomography.
Discussion
In this retrospective and exploratory clinical study, the association between mutations in the MUC5B gene and clinical outcome in patients with rheumatoid arthritis (RA) with interstitial lung disease (RA-ILD) and without ILD were investigated using whole-exome sequencing (WES). The carrier frequency of MUC5B variants was significantly increased in patients with RA-ILD compared with patients with RA without ILD (42.2% vs. 9.1%; p=0.013). Analysis of the associations between MUC5B mutation status and acute exacerbation of RA-ILD or all-cause mortality showed that rare MUC5B variants might be factors associated with disease susceptibility, but were unlikely to be reliable prognostic indicators in patients with RA-ILD.
Previous genome-wide association studies in patients with RA showed that MUC5B gene promoter variants were not indicators of increased risk for the occurrence of RA, but were associated with RA-ILD [20]. However, no such study has been performed on Chinese patients. In this study, the use of WES to search for gene variants in Chinese patients with RA with or without ILD showed similar findings that MUC5B variants were significantly associated with the occurrence of ILD in patients with RA. In this study, missense and frameshift mutations were the most common mutations detected in the patients in the present study, and 10 novel mutations were identified, in addition to the reported promoter mutant, rs35705950. These mutations are enriched in the O-glycosylation region of the protein, which is associated with specific rheological and hydrodynamic properties of mucins [21]. However, although functional studies of MUC5B mutations and their effects on O-glycosylation are limited, it may be hypothesized that the possible mechanism for the effects of MUC5B variants in pulmonary involvement in RA might be explained in three main ways. First, genetically-driven overexpression of the MUC5B gene may contribute to the overproduction of the mucin-5B protein, which interferes with ciliary clearance or disrupts normal lung repair mechanisms. Second, increased levels of mucin-5B may be expressed in the metaplastic epithelium lining the reticular (honeycomb) fibrosis cysts in RA-ILD [9]. Third, the exon variants may affect the charge of mucin-5B in which the low-charge glycated form of mucin-5B leads to the structural anomalies and functional changes in the lung in RA-ILD [22].
The present study included genotype-phenotype correlation analysis, which showed that MUC5B mutations were associated with clinical heterogeneity of RA-ILD. This study was the first to show that MUC5B mutations were more common in patients with a longer history of RA-ILD. Also, MUC5B mutations had a significantly higher frequency in patients with a usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography (HRCT), which is consistent with a previous study [9] in which a variant in the MUC5B gene promoter was associated with a UIP pattern or possible UIP pattern on HRCT (OR 5.0, 95% CI 2.1–12.3; p<0.001). Also, other chronic lung diseases with a UIP pattern on imaging, including chronic hypersensitivity pneumonitis, also showed an increase in MUC5B variants [23]. Although it remains unclear how increased MUC5B expression results in UIP in the lung, MUC5B overexpression has been associated with increased reticular (honeycomb) fibrosis in patients with idiopathic pulmonary fibrosis (IPF) [24,25]. These findings suggest that the MUC5B gene variants might have a potential clinical role as indicators of UIP lung change [9].
Previous studies have shown that variants in the MUC5B gene may be associated with the improved clinical outcomes from lung disease in patients with RA who had rare alleles associated with risk in other genes in different pathways [26]. Studies have also shown that MUC5B variants may improve resistance to infection or enhance tissue repair [10]. However, in the present study, patients with RA-ILD carrying MUC5B variants were prone to early progression, perhaps due to the variable functional consequences of missense variants in this gene. These findings are supported by those of Hozumi et al. [12], who reported that patients with RA-ILD and a UIP pattern on imaging experienced more respiratory-related episodes of hospitalizations and reduced survival when compared with patients with RA without a UIP lung imaging pattern [27–29]. In the present study, patients with a UIP lung imaging pattern and MUC5B gene variants had a worse prognosis. As acute exacerbations of RA-ILD predict a poor outcome [30], further studies are required to determine whether all patients with RA should be evaluated for MUC5B gene carrier status, which may guide early preventive therapy or have prognostic indications for personalized treatment planning.
This study had several limitations, including the small number of patients studied, and there was insufficient power to test associations between variants and individual ILD subsets, for example, with or without acute exacerbations. Also, data on several potential confounding factors were not available, including smoking status, which prevented the exclusion of confounders in the analysis of gene associations. Also, this was a retrospective study that was susceptible to selection bias. Larger, prospective studies investigating MUC5B gene mutations in Chinese patients with RA-ILD are required to refine risk estimates and to determine the clinical implications of the findings of this preliminary exploratory study.
Conclusions
This retrospective and exploratory clinical study aimed to investigate the association between mutations in the MUC5B gene and clinical outcome in patients with rheumatoid arthritis (RA) with interstitial lung disease (RA-ILD) and without ILD were investigated using whole-exome sequencing (WES). The carrier status of MUC5B variants was an indicator of reduced prognosis and increased exacerbations of RA-ILD.
Acknowledgments
The authors thank all the patients and their families for participating in this study.
Footnotes
Source of support: This study was supported by the National Key Research and Development Plan of Precision Medicine Medical Research (Grant No: 2016YFC0905700)
Conflict of interest
None.
References
- 1.Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–67. [PubMed] [Google Scholar]
- 2.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2010;62:1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265–75. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 4.Churg A, Wright JL, Tazelaar HD. Acute exacerbations of fibrotic interstitial lung disease. Histopathology. 2011;58:525–30. doi: 10.1111/j.1365-2559.2010.03650.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim EJ, Collard HR, King TE., Jr Rheumatoid arthritis-associated interstitial lung disease: The relevance of histopathologic and radiographic pattern. Chest. 2009;136:1397–405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics – a large multicentre UK study. Rheumatol. 2014;53:1676–82. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 7.Assayag D, Lubin M, Lee JS, et al. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology. 2014;19:493–500. doi: 10.1111/resp.12234. [DOI] [PubMed] [Google Scholar]
- 8.Juge PA, Borie R, Kannengiesser C, et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J. 2017;49 doi: 10.1183/13993003.02314-2016. pii: 1602314. [DOI] [PubMed] [Google Scholar]
- 9.Juge PA, Lee JS, Ebstein E, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. 2018;379:2209–19. doi: 10.1056/NEJMoa1801562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans CM, Fingerlin TE, Schwarz MI, et al. Idiopathic pulmonary fibrosis: A genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev. 2016;96:1567–91. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peljto AL, Zhang Y, Fingerlin TE, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309:2232–39. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hozumi H, Nakamura Y, Johkoh T, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open. 2013;3:e003132. doi: 10.1136/bmjopen-2013-003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–94. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–88. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 15.Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Resp Med. 2018;6:138–53. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 16.Mak AC, Tang PL, Cleveland C, et al. Brief report: Whole-exome sequencing for identification of potential causal variants for diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2016;68:2257–62. doi: 10.1002/art.39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynisdottir G, Karimi R, Joshua V, et al. Structural lung changes and local anti-citrulline immunity are early features of anti-citrullinated proteins antibodies positive rheumatoid arthritis. Arthritis Rheum. 2014;66:31–39. doi: 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- 18.Spagnolo P, Grunewald J. Genetic determinants of pulmonary fibrosis: Evolving concepts. Lancet Respir Med. 2014;2:416–28. doi: 10.1016/S2213-2600(14)70047-5. [DOI] [PubMed] [Google Scholar]
- 19.Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168:159–66. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 20.Okada Y, Wu D, Trynka G, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersch-Björkman Y, Thomsson KA, Holmén Larsson JM, et al. Large-scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6:708–16. doi: 10.1074/mcp.M600439-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Welsh KG, Rousseau K, Fisher G, et al. MUC5AC and a glycosylated variant of MUC5B alter mucin composition in children with acute asthma. Chest. 2017;152:771–79. doi: 10.1016/j.chest.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley B, Newton CA, Arnould I, et al. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: An observational cohort-control study. Lancet Respir Med. 2017;5:639–47. doi: 10.1016/S2213-2600(17)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang IV, Coldren CD, Leach SM, et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 2013;68:1114–21. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putman RK, Gudmundsson G, Araki T, et al. The promoter polymorphism is associated with specific interstitial lung abnormality subtypes. Eur Respir J. 2017;50 doi: 10.1183/13993003.00537-2017. pii: 1700537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregersen PK. Breathing new life into interstitial lung disease in rheumatoid arthritis. N Engl J Med. 2018;379:2265–66. doi: 10.1056/NEJMe1811767. [DOI] [PubMed] [Google Scholar]
- 27.Nurmi HM, Purokivi MK, Kärkkäinen MS, et al. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm Med. 2016;16:107. doi: 10.1186/s12890-016-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya Y, Takayanagi N, Sugiura H, et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J. 2011;37:1411–17. doi: 10.1183/09031936.00019210. [DOI] [PubMed] [Google Scholar]
- 29.Song JW, Lee HK, Lee CK, et al. Clinical course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:103–12. [PubMed] [Google Scholar]
- 30.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–20. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]