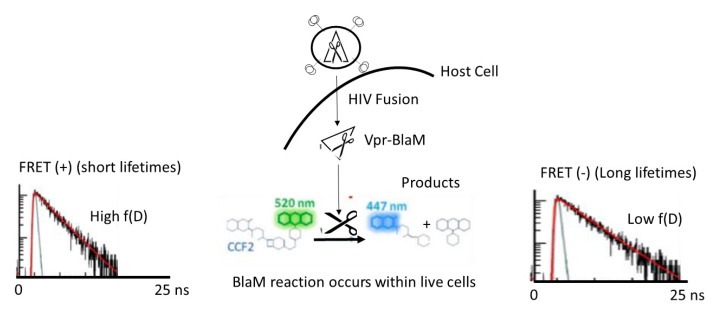
Figure 1.
Schema of the β-lactamase (BlaM) assay applied to study human immunodeficiency virus type 1 (HIV-1) fusion utilizing intensity-based Förster resonance energy transfer (FRET) approaches and FRET fluorescence lifetime imaging microscopy (FLIM). HIV-1 pseudoparticles bearing the Vpr-BlaM are exposed to live cells expressing CD4 and co-receptors so that the HIV-1 particles are allowed to fuse with the host membrane and release their capsid into the host cytosol. The BlaM enzyme reaches the CCF2-AM FRET biosensor composed of hydroxycoumarin linked to fluorescein by the BlaM recognition domain. Once the BlaM enzyme starts the catalytic reaction, CCF2-AM is cleaved into hydroxycoumarin (donor) and fluorescein (acceptor) and FRET is disrupted. When employing FLIM, one can apply a two-exponential method that takes into account the situation in which not all fluorophore is engaged in FRET. A change from green to blue is also observed when looking at the visible emission spectra of CCF2-AM during the BlaM enzymatic reaction. The green emission (~520 nm) when exciting hydroxycoumarin at 405 nm comes from FRET; when CCF2-AM is cleaved hydroxycoumarin emits in the blue (~447 nm).