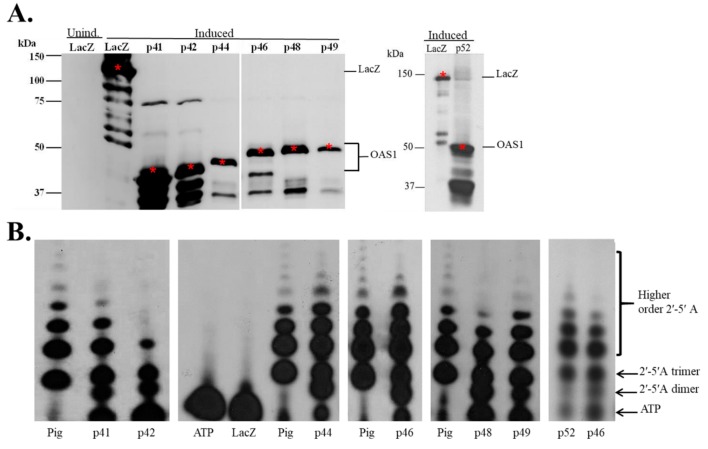
Figure 2.
Bacterial expression of hOAS1 isoform proteins and analysis of their ability to produce 2-5A. (A) Seven hOAS1 isoform fusion proteins and LacZ protein were expressed individually in bacteria. After isopropyl β-d-1-thiogalacto-pyranoside (IPTG) (1 mM) induction, bacteria were lysed and proteins in the crude bacterial lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane and immunoblotted with anti-V5 antibody. All of the OAS1 isoforms except for p52 were run on the same gel. A lysate from uninduced bacteria transformed with a plasmid containing the LacZ gene was used as a negative control. The full-length proteins are indicated by a red asterisk. OAS1 degradation bands were detected on the gel below the full-length proteins. (B) After protein purification on a Talon metal affinity column, each human OAS1 isoform (22 μL) was incubated with α32P-adenosine triphosphate (ATP) and poly(I:C) for 18 h at 30 °C. LacZ protein plus α32P-ATP (Lane 5) and α32P-ATP alone (no protein) (Lane 4) were used as negative controls. Purified Escherichia coli (E. coli)-expressed pig OAS1 (0.5 μg) was used as a positive control. Two µL of each reaction were electrophoresed on a 20% polyacrylamide/8M urea denaturing gel and radiolabeled 2-5A was visualized by autoradiography. Separate gels used to analyze the 2-5A are indicated by spaces.