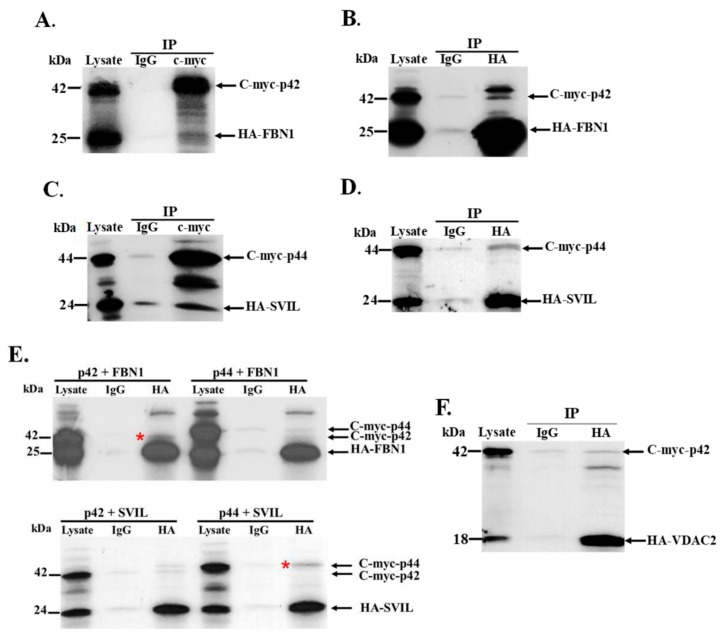
Figure 5.
Analysis of the interaction between human OAS1 p42 and p44 isoforms and their respective binding partners in vitro. Wheat germ extract was used for the in vitro transcription and translation of c-myc tagged full length human OAS1 isoforms and of HA-tagged FBN1 (188 aa), SVIL or voltage-dependent anion channel (VDAC) peptides in the presence of [35S]-methionine. Reciprocal pull-down assays were performed with anti-HA and anti-c-myc antibodies. Non-specific IgG was used as the negative control. The protein complexes were resolved by SDS-PAGE and detected by autoradiography. (A) HA-FBN1 peptide and full-length c-myc-OAS1 p42 immunoprecipitated with anti-c-myc antibody. (B) C-myc-OAS1 p42 and HA-FBN1 peptide immunoprecipitated with anti-HA antibody. (C) HA-SVIL peptide and c-myc-OAS1 p44 immunoprecipitated with anti-c-myc antibody. (D) C-myc-OAS1 p44 and HA-SVIL peptide immunoprecipitated with anti-HA antibody (E) (top panel) Either C-myc-OAS1 p42 or p44 and HA-FBN1 peptide immunoprecipitated with anti-HA antibody and (bottom panel) either C-myc-OAS1 p42 or p44 and HA-SVIL peptide immunoprecipitated with anti-HA antibody. The hOAS1 isoform specifically immunoprecipitated is indicated with a red asterisk. (F) C-myc-OAS1 p42 and HA-VDAC2 peptide immunoprecipitated with anti-HA antibody. IgG: non-specific antibody control; IP: immunoprecipitation.