Abstract
Mania is a serious neuropsychiatric condition associated with significant morbidity and mortality. Previous studies have suggested that environmental exposures can contribute to mania pathogenesis. We measured dietary exposures in a cohort of individuals with mania and other psychiatric disorders as well as in control individuals without a psychiatric disorder. We found that a history of eating nitrated dry cured meat but not other meat or fish products was strongly and independently associated with current mania (adjusted odds ratio 3.49, 95% confidence interval (CI) 2.24–5.45, p < 8.97 × 10−8). Lower odds of association were found between eating nitrated dry cured meat and other psychiatric disorders. We further found that the feeding of meat preparations with added nitrate to rats resulted in hyperactivity reminiscent of human mania, alterations in brain pathways that have been implicated in human bipolar disorder, and changes in intestinal microbiota. These findings may lead to new methods for preventing mania and for developing novel therapeutic interventions.
Introduction
Mania is an abnormal mood state and the defining characteristic of bipolar disorder (BPD), a serious mental illness associated with significant morbidity and mortality. Genome-wide association studies (GWASs) have identified several genetic risk factors for BPD. However, estimated heritability for BPD from genetic studies falls far short of heritability as estimated from twin and family studies. This heritability gap apparent in BPD and other neuropsychiatric disorders points to the role of the environment in mediating and propagating neuropsychiatric disease [1]. In turn, it suggests that modification of the environment may have preventative and therapeutic effects [1].
Diet is increasingly recognized as a source of environmental factors that may contribute to risk for BPD and other neuropsychiatric disorders [2–4]. Mechanisms by which diet is proposed to alter risk of mania and other mood disorders include immune-system modulation through the consumption of immune-reactive compounds such as wheat gluten [5], interaction between brain metabolism and ingested fatty acids [6], neurotoxicity from dietary exposure to trace heavy metals [7], metabolism of neuroactive intermediates such as tryptophan and kynurenine [8], manipulation of the gut microbiome and gut–brain axis [9], and induction of oxidative and nitrosative stress in the brain [10]. However, specific parameters of the relationship between dietary exposures and risk of mania require further investigation.
To better understand the potential impacts of environmental exposures on psychiatric conditions, we measured exposure to selected food products in a cohort of 1101 individuals with and without psychiatric disorders. This study was part of an ongoing cohort study of individuals with psychiatric disorders initiated in 2001 with dietary data collected since 2007 [11]. Food products were selected for inquiry based on potential exposures to infectious agents and other foodborne environmental factors. We found unexpectedly that dietary exposure to nitrated cured meats was associated with a markedly increased rate of being hospitalized with acute mania. To better assess cause and effect in a controlled study, we modeled this relationship in rats. Both feeding of commercially available nitrated meat and a purified fixed-nitrate diet resulted in mania-like changes to locomotor and novelty-associated hyperactivity. These behavioral changes were accompanied by alterations in hippocampal gene expression and gut microbiota.
While dietary exposure to nitrated cured meat is implicated in several other disorders including various cancers, asthma, and chronic obstructive pulmonary disease, the mechanisms underlying these associations are poorly understood [12, 13]. Together, our findings also link dietary nitrate exposure to neuropsychiatric alterations and begin to elucidate potential mechanisms therein.
Methods
Human studies
Human study populations
The study population of individuals with psychiatric disorders was recruited from a cohort of individuals receiving psychiatric care at the Sheppard Pratt Health System and affiliated agencies in the Baltimore, MD, USA region. The study period ranged from January 1, 2007 to March 30, 2017. Methods of recruitment and evaluation of participants have been previously published [14, 15] and are outlined below. The studies were approved by the Institutional Review Boards of the Sheppard Pratt Health System and the Johns Hopkins School of Medicine. Informed consent was obtained from each participant.
Inclusion criteria
The inclusion criterion for the mania participants was current admission to an inpatient or day hospital program for symptoms of mania or hypomania [5]. Participants with mania could have a diagnosis of any one of the following: Bipolar I disorder, single manic episode; Bipolar I disorder, most recent episode manic; Bipolar I disorder, most recent episode mixed; Bipolar II disorder, most recent episode hypomanic; or Schizoaffective disorder, bipolar type (manic, hypomanic, or mixed state). Individuals with bipolar depression were recruited from persons admitted to an inpatient or day hospital unit for symptoms of depression and a diagnosis of bipolar I or II disorder, most recent episode depressed [15]. Individuals with major depressive disorder were also drawn from the hospital setting and had a diagnosis of major depressive disorder, recurrent or single episode. Individuals with schizophrenia were recruited from local psychiatric treatment centers as well as from inpatient and day hospital programs. The inclusion criterion for this group was a diagnosis of schizophrenia or schizoaffective disorder. Individuals without a history of psychiatric disorder were recruited from posted announcements at local health-care facilities and universities located in the same geographic area as the psychiatric participants [16]. These control individuals were enrolled after they were screened to rule out the presence of a current or past psychiatric disorder with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV Axis I Disorders—Non-patient Edition [17]. Participants in all groups met the following additional criteria: age 18–65 years (except the control participants who were aged 20–60 years [18]); proficient in English; absence of any history of intravenous substance abuse; absence of mental retardation by history; absence of human immunodeficiency virus infection; absence of serious medical disorder that would affect cognitive functioning; absence of a primary diagnosis of alcohol or substance use disorder. The diagnosis of each psychiatric participant was established by consensus of the research team based on the Structured Clinical Interview for DSM-IV Axis 1 Disorders—Patient Edition [17] and available medical records. For the purposes of this study, participants were only included in one psychiatric group with the order of selection: mania (N = 217), bipolar depression (N = 91), major depressive disorder (N = 79), or schizophrenia (N = 371). In addition, a total of N = 343 control individuals without a psychiatric disorder were enrolled. Methods for the recruitment and assessment of control individuals have been previously described [19].
Data collection
Demographic data were collected from all participants. All participants were queried concerning current cigarette smoking and their cognitive functioning was assessed with the Repeatable Battery for the Assessment of Neuropsychological Status [20]. Clinical information about psychiatric history, drug/alcohol abuse, and current medications was obtained from all cases via medical records and self-report. Symptoms at the time of the assessment were measured by the Brief Psychiatric Rating Scale and the Young Mania Rating Scale as previously described [21]. Detailed demographic data relating to the study population are presented in Table S1.
Food exposures
Food exposures were assessed by a questionnaire developed and administered by research staff. This questionnaire asked the participants to indicate whether or not they had eaten certain types of food. Data were not collected regarding the amount of food consumed or the timing of consumption. Questions addressed to the participants included “Have you ever eaten locally procured dry cured meat”? “Have you ever eaten undercooked fish such as rare tuna?”; “Have you ever eaten raw fish such as sushi or sashimi?”; and “Have you ever eaten undercooked meat?” Starting January 1, 2015, additional questions were asked of individuals who answered affirmatively to the question “Have you ever eaten locally procured dry cured meat?” These questions inquired of the participant whether s/he consumed the dry cured meat in the form of beef jerky, turkey jerky, meat sticks (generally indicated as “Slim Jim”), prosciutto, salami, or “other”. These questions were added following an interim analysis that indicated an association between eating cured meat and diagnostic group.
Statistical analyses
The relationship between diagnostic group and exposure to individual items on the food questionnaire was determined through the use of several logistic regression models controlling for age, gender, race, current cigarette smoking, body mass index, and socioeconomic status, the latter estimated by level of parental education. Missing data relating to parental education (N = 13) and body mass index (N = 105) were added by multiple imputation. Multivariate logistic regression models were employed to define the independent effect of each covariate in a model containing all five relevant exposures (dry cured meat, undercooked meat, raw meat, undercooked fish, raw fish), as well as the independent effects of each exposure in the presence of other exposures. Similarly, the relationships between exposures and demographic and clinical variables within the group of individuals with mania were determined utilizing logistic regression models. For each of the above models, the calculated probability values were adjusted to account for N = 5 multiple comparisons through the use of Bonferroni correction. Similar analyses were performed analyzing the type of cured meat. Owing to the smaller sample size and more limited statistical power, these analyses were limited to the mania and control groups and included only age, gender, race, and parental education as covariates. These statistical analyses were performed by the use of STATA v12 (Stata Corporation, College Station, TX).
Animal studies
Diets
The diet preparations used in the animal studies are detailed in the Supplemental Methods and Tables S2–S4. Listed levels of nitrate and other nutrients were measured by high-performance liquid chromatography or other chromatographic methods using previously described methods [22].
Experimental design
Experiment 1
Twenty-four adult male Sprague-Dawley rats (8–10 weeks on arrival, Charles River, Raleigh, NC, USA) were individually housed in standard polycarbonate cages on a 12h:12h light cycle (lights on 0700 hours) in a climate-controlled room. Rats were acclimated to the laboratory environment with ad libitum access to water and standard chow (2018 Teklad rodent diet) for 1 week prior to beginning diet experiments. Following 1 week of acclimation, rats were randomly assigned to one of the two weight-matched groups: ad libitum access to standard chow only (“Chow2018” group, n = 12) or ad libitum access to standard chow supplemented every other day with 14 g of the BJ-Comm commercial beef jerky preparation (“BJ-Comm” group, n = 12). Body weight, 2-day chow intake, and 2-day jerky intake were measured every other day for the duration of the study, corresponding with the days of jerky supplementation. Rats underwent behavioral testing beginning on day 15 of diet (BJ-Comm or Chow2018) exposure, with diet exposure continuing throughout the behavioral testing period until rats were killed by rapid decapitation on day 35.
Experiment 2
In a second cohort, 30 adult male Sprague-Dawley rats were randomly divided into three weight-matched groups following 1 week of acclimation and provided ad libitum access to Chow2018 (n = 10), Chow2018 supplemented with BJ-Comm (14 g every other day, n = 10), or Chow2018 supplemented with a custom-formulated cured meat product without preservatives or nitrate added (14 g every other day, “BJ-NoPreserve” group, n = 10). Study timeline, housing, behavioral testing, and termination were the same as those described in Experiment 1.
Experiment 3
In a third cohort, 30 adult male Sprague-Dawley rats were randomly divided into three weight-matched groups following 1 week of habituation and provided ad libitum access to one of the following diets: (1) a standard AIN-93M rodent diet (“ChowAIN” group, n = 10); (2) a modified isocaloric AIN-93M 35% kcal protein beef diet (“ChowAIN+Beef” group, n = 10); or (3) a modified isocaloric AIN-93M beef and nitrate diet with sodium nitrate added to match the calculated nitrate exposure in Experiments 1 and 2 (“ChowAIN+Beef+Nitrate” group, n = 10). Body weight and 2-day diet intake were measured every other day through the duration of the experiment at the same time points as described in Experiments 1 and 2. Study timeline, housing, behavioral testing, and termination remained the same as those described in Experiments 1 and 2.
Behavioral tests
Locomotor activity
Locomotor activity monitoring began on day 15 of diet exposure in each experiment. On the day of testing, rats were moved into the testing room in their home cages to habituate for 1 h. The testing room was maintained on the same 12 h:12 h light cycle as the housing facility. Two hours prior to the start of the dark period, rats were individually placed into 40 × 40 × 30 cm3 clear plastic boxes attached to a VersaMax Activity Monitoring System (Omnitech Electronics, Inc., Columbus, OH) and locomotor activity (distance traveled) was measured over a 22-h period. No food or water was provided during the testing period so as to not confound the longer-term effects of diet supplementation on locomotor activity with potential acute effects of in-test diet access [23]. Following 22 h, rats were returned to their home cages and housing facility, provided diet (as per experimental group) and water, and checked for physical wellbeing. Data were collated into 30-min bins for the 22-h testing period. Variables analyzed were 22-h total activity (distance traveled), dark period activity (12 h), light period activity (10 h), and initial 2-h activity upon introduction to the testing chamber. Rats were given at least 2 days of rest following activity monitoring before the novel environment test below (Experiments 2 and 3 only).
Novel environment test
Given human studies suggesting that exposure to novelty may precipitate manic episodes, we measured activity in response to a novel environment in Experiments 2 and 3 [24, 25]. On the day of testing, each rat was brought to a novel testing room in its home cage and immediately placed into a clean activity monitor chamber for 10 min. The chamber was cleaned with 70% ethanol between each animal to remove odorant cues. Testing was conducted in the middle of the light cycle period, with testing order alternating between diet groups. Data (distance traveled) were collated into 1-min bins for analysis.
Statistical analysis of behavioral tests
Statistical analyses were completed using Statistica 7 (StatSoft, Tulsa, OK). Data are expressed as means ± SEM. In Experiment 1, differences between groups were assessed via Student’s t test. In Experiments 2 and 3, differences between groups were assessed using one-way or mixed model analysis of variance (ANOVA) with “Diet” as a between-subject factor and “Time” as the repeated factor followed by Tukey’s post-hoc analysis. In Experiment 3, the ChowAIN+Beef+Nitrate group was compared to the ChowAIN and ChowAIN+Beef groups by one-way ANOVA analysis followed by planned comparison t test. For all tests, p < 0.05 was considered significant. All animals were included in the behavioral testing and analysis. Given the size and expected variance of the behavioral measures based on previous experiments, group sizes of 10–12 rats per diet exposure group gave us >90% chance of finding statistically significant (p < 0.05, two-tailed) differences.
Microarray analysis
Hippocampal RNA transcript levels were quantified by microarray analyses on the Affymetrix Rat Clariom S Array GeneChip, which interrogates all known exons of >23K coding transcripts. RNAs were amplified into cDNA and biotinylated by in vitro transcription with Affymetrix reagents, using the 3’ IVT Pico Target Labeling Kit protocol as described in the Affymetrix manual (http://www.affymetrix.com/support/technical/product updates/wt_1_1_assay.aff). Biotinylated cDNAs were purified, fragmented, and subsequently hybridized to the arrays. Detailed descriptions of the methods for sample preparation, measurement, and statistical analysis are provided in supplementary material. The microarray results have been deposited in the GEO repository of NCBI as study number GSE112510,
Microbiota analyses
To assess the bacterial composition of the rats’ small intestine, ileal and jejunal fecal contents obtained following the completion of Experiment 3 were collected for 16S rRNA gene sequencing. Genomic DNA was isolated from ileal and jejunal samples obtained from rats fed the ChowAIN+Beef+Nitrate (33.9 mg/kg nitrate) and ChowAIN+Beef (0 mg/kg nitrate) preparations, along with 17 blank extraction and library preparation control samples, using the Quick DNA Fecal Mini Kit (Zymo Research, Irvine, CA). Libraries were prepared targeting the v3v4 region on the 16S rRNA gene using the standard Illumina 16S Metagenomic library preparation guide. Each library concentration was determined using Quibit normalized to 4 nM and 5 μl of each library was pooled together, along with blank control library samples, for sequencing. The pooled library mix was denatured with 0.2 N NaOH and subsequently diluted to 10 pM using HT1 (hybridization buffer) supplied by Illumina. Prior to loading, the library was spiked with a 10% 20 pM PhiX standard. Sequencing was performed on the MiSeq Illumina sequencing platform using a 2× 300 PE Sequencing Reagent Kit (Cat # MS-102–3003 Illumina, San Diego, CA).
Detailed methods for tissue collection, normalization of microarray analyses, and analysis of intestinal microbiota are presented in supplementary materials. All protocols used for the animal studies were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Results
Human studies
A history of eating cured meat preparations was associated with significantly increased odds of being in the mania group (Adjusted odds ratio 3.49, 95% confidence interval (CI) 2.24–5.45, p < 9 × 10−8 adjusted for age, race, socioeconomic status, body mass index, cigarette smoking, and multiple comparisons) (Fig. 1). Multiple logistic regression models containing all relevant food exposures indicated that eating cured meat preparations was independently associated with mania (Adjusted odds ratio 3.31, 95% CI 2.04–5.3, p < 3.3 × 10−6 adjusted for age, sex, race, socioeconomic status, body mass index, cigarette smoking, and multiple comparisons) while the other food associations with mania were non-significant (Table S5). A history of eating cured meat preparations was not associated with a diagnosis of schizophrenia or schizoaffective disorder, BPD, bipolar depression, or major depressive disorder (all p > 0.05). Within the group of individuals with mania, stepwise regression analysis indicated that a history of eating a cured meat product was not associated with age, race, sex, cigarette smoking, body mass index, socioeconomic status, symptom rating scores, or a history of drug or alcohol abuse (all p > 0.05 adjusted for multiple comparisons).
Fig. 1.
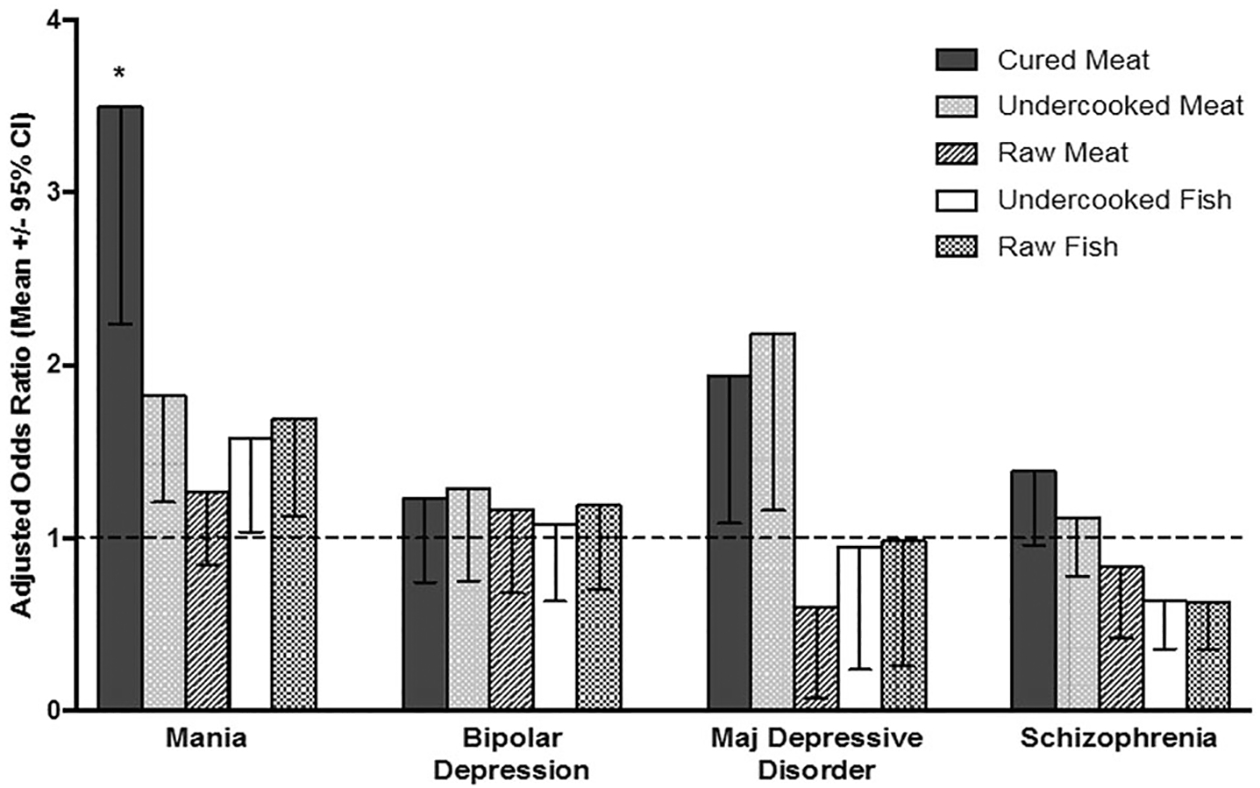
Adjusted odds ratios associated with a food exposure and having the indicated psychiatric diagnosis as compared to controls. Adjusted odds ratios are calculated by multiple logistic regression and adjusted for age, sex, race, socioeconomic status, body mass index, current cigarette smoking, and multiple comparisons. *p < 9 × 10−8
Detailed data on cured meat consumption were also available from 42 individuals with mania enrolled after January 1, 2015 and 35 control individuals enrolled during the same time period (Table S6). These data were based on additional questions that were added following an interim analysis, indicating a differential response to the survey question relating to “dry cured meat” by diagnostic group, with the goal of obtaining more detailed information regarding the exposure. Analysis of this dataset indicates increased odds of consuming meat sticks (adjusted odds ratio 5.15, 95% CI 1.71–15.2, p = 0.003), beef jerky (adjusted odds ratio 4.81, 95% CI 1.48–14.3. p = 0.006), or turkey jerky (adjusted odds ratio 3.54, 95% CI 1.11–11.3, p = 0.032; all adjusted for age, sex, race, and socioeconomic status and diagnostic group. In contrast, consuming prosciutto or salami, cured meats prepared through dehydration, did not influence the odds of being in the mania group (p > 0.05).
Animal studies
Ingestion of a commercially available cured meat product with added nitrates (“BJ-Comm”) in addition to standard rodent chow (“Chow2018”) for 2 weeks resulted in locomotor hyperactivity over a 22-h longitudinal activity assay, with greater activity as measured by distance traveled within the first 2 h of activity monitoring, during the light cycle period, and over the full 22-h test period (Fig. 2a). This behavioral outcome was replicated in a second cohort of rats in which consumption of the same BJ-Comm product led to increased activity during the first 2 h of monitoring and over the light cycle period (Fig. 2b). Rats exposed to BJ-Comm also demonstrated greater novelty-induced locomotor hyperactivity compared to the control diet group (Fig 2d, e).
Fig. 2. a–g.
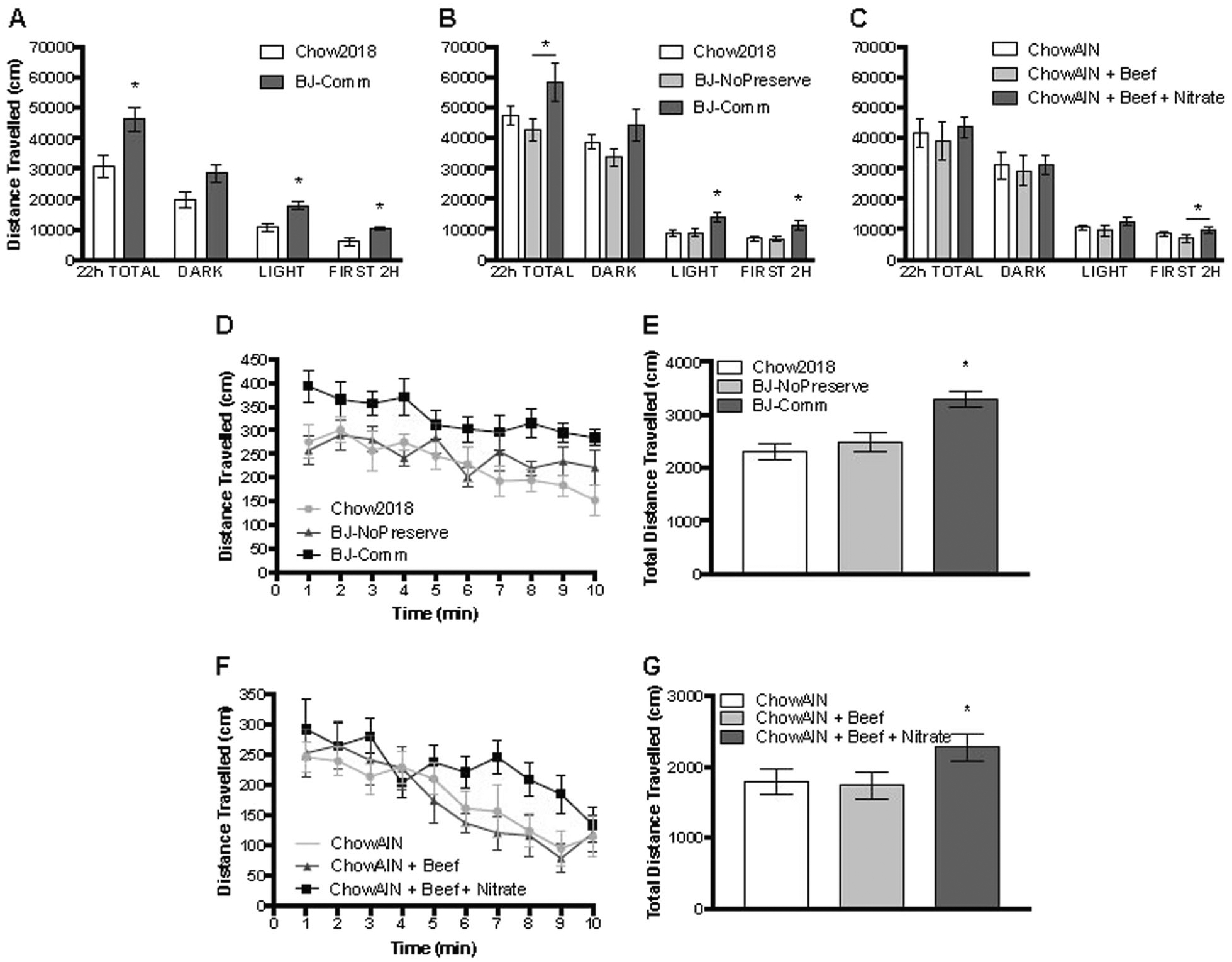
Rats consuming nitrated cured meat or a purified beef-based diet dosed with sodium nitrate exhibit mania-like hyperactivity. a–c Longitudinal activity monitoring. Rats consuming nitrated cured meat exhibit locomotor hyperactivity in comparison to rats consuming standard chow only or cured meat prepared with no preservatives. Rats consuming a purified beef-based diet with added sodium nitrate display locomotor hyperactivity within the first 2 h of a 22-h longitudinal activity monitoring test in comparison to rats consuming an identical purified beef-based diet without added sodium nitrate. d–g Activity in a novel environment. Rats consuming nitrated cured meat or a purified beef-based diet dosed with sodium nitrate exhibit novelty-induced hyperactivity over a 10-min period compared to rats consuming either a purified standard chow diet or an identical purified beef-based diet without added sodium nitrate. *p < 0.05 compared to the indicated groups
In contrast, consumption of a custom formulated cured meat product prepared without added nitrates (“BJ-NoPreserve”) did not induce behavioral changes in total locomotor activity (Fig. 2b) or novelty-induced activity (Fig. 2d, e). All rats that were fed a cured meat product (BJ-Comm or BJ-NoPreserve) in addition to ad libitum rodent chow consumed a similar amount of cured meat product and decreased their intake of the control diet such that there were no differences in total energy intake among the groups in any of the experiments (Figure S1).
We tested the effect of nitrate additive alone on behavioral hyperactivity by using a purified diet (“ChowAIN”, 13% kcal protein) with sodium nitrate added in fixed amount to replicate the nitrate intake seen in Experiments 1 and 2 (Table S4; Figure S2). Rats consuming a nitrate-added high-beef diet (“ChowAIN+Beef+Nitrate”, 35% kcal protein, 33.9 mg/kg nitrate) displayed locomotor hyperactivity in the first 2 h of observation as compared to rats consuming a non-nitrated high-beef protein control diet (“ChowAIN+Beef”, 35% kcal protein, 0 mg/kg nitrate) (Fig. 2c). The rats fed the ChowAIN+Beef+Nitrate diet also displayed increased novelty-induced hyperactivity compared to both the ChowAIN standard control and ChowAIN+Beef protein control groups (Fig. 2f, g).
Comparison of gene expression in the hippocampus of rats fed the ChowAIN+Beef+Nitrate diet to those fed the non-nitrated ChowAIN+Beef protein control diet indicated dysregulation in several functional pathways. Specific pathways with significant levels of dysregulation include those relating to serotonin receptor signaling, nuclear factor (NF)-κB signaling, bacterial pattern recognition, and sphingosine-1-phosphate signaling (Fig. 3a–d, Figure S3, Table S7).
Fig. 3. a–d.
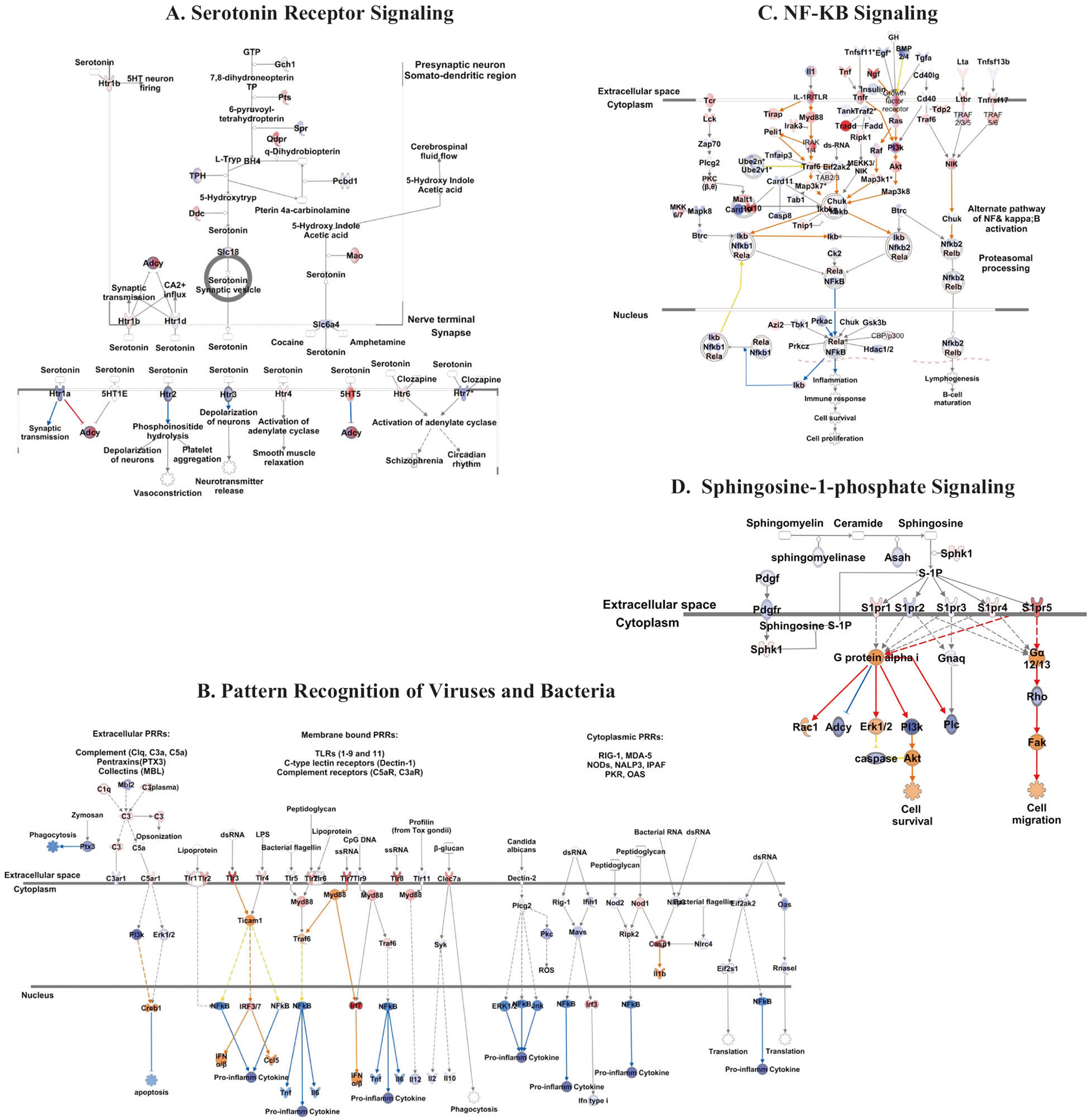
Rats consuming a purified beef-based diet dosed with sodium nitrate exhibit alterations in gene expression in the hippocampus. Ingenuity pathways depicted with member genes are color-coded to show expression fold change within the hippocampus of rats fed ChowAIN+Beef+Nitrate (33.9 mg/kg nitrate) as compared to rats fed ChowAIN+Beef (0 mg/kg nitrate). Genes demonstrating >2 SD fold changes are highlighted. The numbers shown represent the log ratio of expression change between the two groups. Additional explanation of the color coding can be seen in supplemental figure S2. Individual genes that comprise the pathways are depicted in supplemental Table S7. The pathways depicted represent a serotonin receptor signaling (p < 0.0009), b role of pattern recognition receptors in recognition of bacteria and viruses (p < 0.001), c NF-κB signaling (p < 0.009), and d sphingosine-1-phosphate signaling (p < 0.009)
We also examined the effect of the diets on the bacterial microbiota of the small intestine. The alpha diversity measures such as the Pileou’s evenness and Shannon diversity indices did not differ significantly between rats fed ChowAIN+Beef+Nitrate and those fed ChowAIN+Beef. However, as depicted in Fig. 4, there were increased levels of bacteria of the taxa Lachnospiraceae (z = 3.04, p = 0.002) and Erysipelotrichales (z = 2.60, p = 0.009) in the ChowAIN+Beef+Nitrate rats compared to the ChowAIN+Beef rats.
Fig. 4.
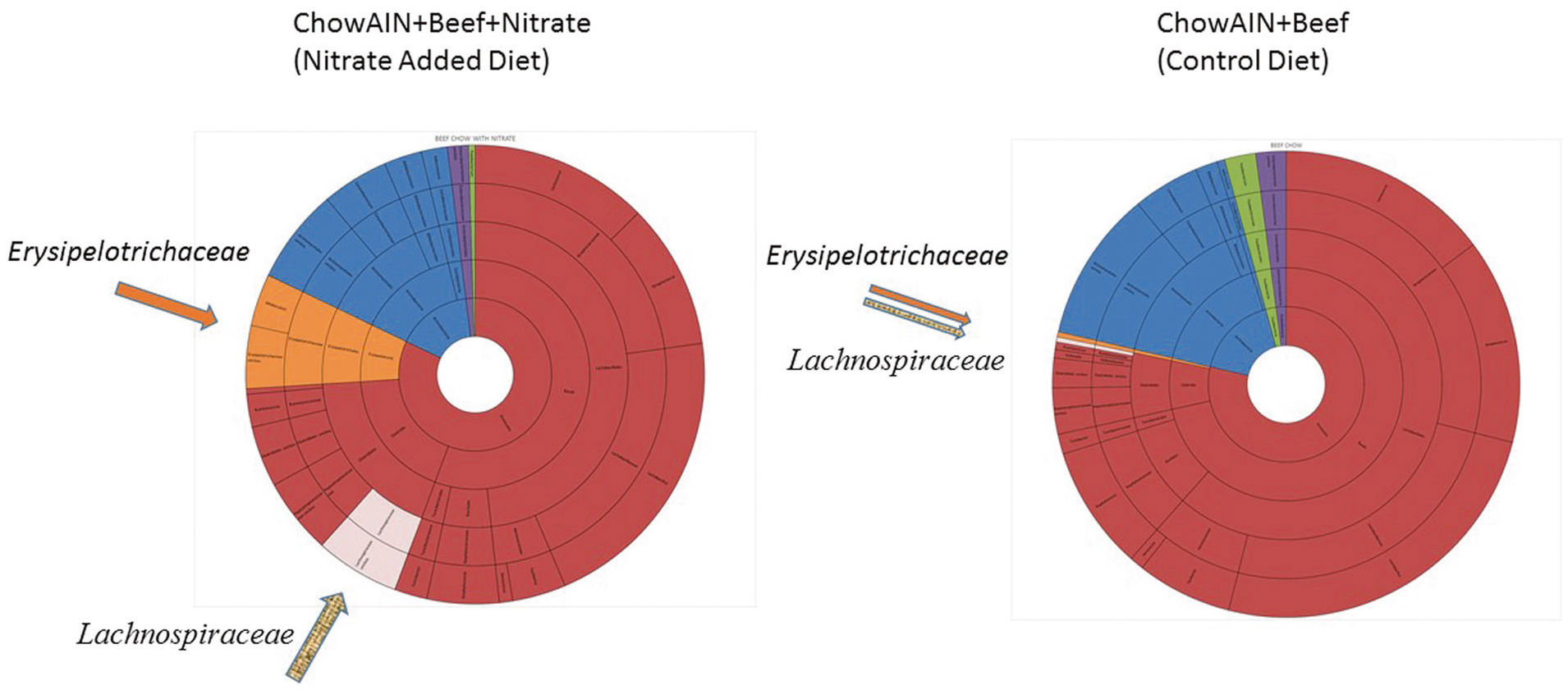
Comparison of the microbiota of rats fed control and nitrate-added diets. Arrows and coloring indicate the microbial taxa that differ significantly between the groups: Lachnospiraceae (pink, p < 0.002) and Erysipelotrichales (orange, p < 0.009). Phyla shown are Firmicutes (brown), Actinobacteria (blue), Fusobacteria (green), and Proteobacteria (purple)
Discussion
We found that a history of eating cured meat products with added nitrates is associated with increased odds of acute mania in humans. The cured meat products were generally in the form of meat sticks, beef jerky, and turkey jerky, which are cured meat products generally prepared with added nitrates [26]. The association between mania and exposure to cured meat products was independent of potentially confounding factors, such as age, race, sex, socioeconomic status, body mass index, or cigarette smoking. Also, associations with mania were not found with other exposures such as raw or undercooked meat or fish, making it unlikely that demographic differences or recall bias were the basis of our findings. Lower odds ratios were found between eating different cured meat preparations and other psychiatric diagnoses, suggesting some degree of common exposures among these different psychiatric disorders.
Cause and effect associations between dietary exposures and behavior in humans can be difficult to establish with certainty due to the possible effects of unmeasured exposures, environmental cofactors, unmeasured differences in socioeconomic status, and other demographic variables. We thus further interrogated the human results by feeding meat products containing added nitrate to wild-type rats and assessing mania-like behavioral changes in locomotor activity patterns and alterations in hippocampal gene expression and gut microbiome.
We selected Sprague-Dawley rats over other potential model organisms for their extensive use in neuropsychiatry and behavioral research, allowing comparison with other studies in the field [27]. Additionally, in comparison to mice, (1) rats display greater and more consistent feeding, allowing for a more accurate assessment of intake and replicability of nitrate dose, (2) the rat brain shares increased homology with the human brain, and (3) rats are suggested to exhibit a decreased stress response to human handling in behavioral experiments, important in interpreting behavioral analyses [28].
We sought in the rat model to evaluate clinically relevant levels of cured meat and nitrate intake to maintain translatability. In the experiments in which cured meat was provided, rats consumed approximately 15–20% of their daily caloric intake from cured meat (Figure S1). Considered in terms of an adult human diet, this percentage is less than what the average human adult consumes from nonmeal snacks [29]. Regarding nitrate consumption, it is estimated that in the US mean dietary exposure to nitrate ranges from 53 to 367 mg/person/day [30]. Across all studies, rats consuming nitrated cured meat product or fixed-nitrate chow averaged 1.84–2.45 mg/day nitrate intake. In a 75-kg person, this would correspond to 154.3–183.8 mg/person/day, within the range of average human exposure. Figure S2 depicts 2-day nitrate consumption over the experimental period for BJ-Comm rats and ChowAIN+Beef+Nitrate rats to illustrate the consistency of nitrate exposure between experiments and across the experimental periods.
Consistent with the human results, consumption of meat products with added nitrate resulted in mania-like hyperactivity in rats across the three experimental cohorts (Fig. 2). In Experiments 1 and 2, rats consuming the commercially available cured meat product BJ-Comm displayed both overall and novelty-induced hyperactivity in comparison with rats consuming the BJ-NoPreserve non-nitrated cured meat product or the Chow2018 control diet alone. In Experiment 3, rats consuming the purified fixed-nitrate ChowAIN+Beef+Nitrate diet exhibited hyperactivity within the first 2 h of the 22-h locomotor activity test and novelty-induced hyperactivity within the novel environment test compared to rats consuming the ChowAIN+Beef non-nitrated protein control diet. It is of note that the rats fed the ChowAIN+Beef+Nitrate diet appear to have a less robust hyperactivity phenotype compared to the rats fed the commercial BJ-Comm diet, suggesting that, while dietary nitrate consumption does appear to result in hyperactivity, it may not completely account for the association between nitrated cured meat and mania or mania-like behavior in the human or animal studies. Future studies would be needed to determine whether other compounds found in cured meat could contribute to or exacerbate the observed nitrate effect.
Mania in humans is complex and heterogeneous, with a range of symptoms and presentations therein that often cloud clinical categorization. This diversity of presentations is reflected in the animal literature, in which different proposed animal models of mania have been shown to exhibit seemingly contradictory characteristics, such as both increased and decreased anxiety-like behavior [31]. The unclear etiology of mania and frequent presence of psychiatric co-morbidities further complicates attempts to define and model the condition [32]. Yet across the heterogeneous spectrum of manic presentations, psychomotor agitation has been suggested as a common denominator. Psychomotor agitation is present in 90% of human manic episodes—more than any other symptom including other DSM V-defined core features of mania—leading to the widely cited hypothesis that hyperactivation may be the core underlying feature of the state [33, 34]. In addition, motor hyperactivity and increased exploratory behavior are translational measures that have been shown to have cross-species relevance, including in human patients [33]. Given this rationale, we selected to focus our behavioral assessments on locomotor activity as a characteristic and translatable set of endophenotypes seen in human mania.
Animal models of mania have been shown to have differential levels of activity compared to controls whether assessed during dark cycle, the predominant time of activity for rodents, which are nocturnal, or light cycle. Additionally, published models of mania have shown disrupted circadian rhythms in “manic” rodents, a feature also seen in human mania [35]. Thus it has become standard to evaluate activity over whole-day or even multi-day periods [36]. As our locomotor activity test was done without food to minimize the potential effect of in-test access to the experimental diets and resulting food cues, which have been shown to affect locomotor behavior, we limited the test to 22 h, a fasting length that does not appear to cause undue stress to the animals as has been assessed by serum corticosterone levels, yet allows us to evaluate longitudinal activity over both light and dark cycle [37]. While we were not able to assess sleep bouts in this test, it is interesting to note that the hyperactivity in our nitrate-fed rats appears to be driven by the light cycle period, when rats undergo the majority of their sleep. Future studies could specifically assess whether these rats show alterations in circadian periodicity. Given the observation from human patients that exposure to novelty may precipitate manic episodes in patients with BPD, the novel environment test was designed to evaluate locomotor behavior in response to novelty [24, 25]. The overall locomotor hyperactivity and novelty-induced hyperactivity seen in our rats is consistent with human patients, who have been shown to exhibit both increased motor activity overall and increased exploratory activity when in a novel environment [38, 39].
There are a select group of other rodent behavioral assays that have also demonstrated direct clinical relevance to human mania, including prepulse inhibition to assess deficits in sensorimotor gating, cocaine and amphetamine self-administration to assess pleasure-seeking behavior, and behavioral characterization for predictive validity following lithium treatment [40]. While in this study we limited our behavioral analyses—using a set of tests that have demonstrated validity and translational relevance but are also non-invasive and relatively low-stress so as to minimize potential influence on our molecular analyses—we hope in future studies to also examine these endophenotypes in our animal models.
Rodent consumption of the purified ChowAIN+Beef+Nitrate diet was also associated with altered transcription of hippocampal signaling pathways (Fig. 3, Figure S3, Table S7). Many of the pathways found to be altered in ChowAIN+Beef+Nitrate rats overlap with genetic polymorphisms and pathways altered in some human patients with mania. For example, polymorphisms of Toll-like receptor (TLR) genes [41] and altered expression of TLRs in the brain [42] have been found in individuals with BPD. Similarly, NF-κB has been found to be dysregulated in BPD and postulated to exert its effect by the transcriptional activation of TLRs [43]. In addition, variants in the genes encoding serotonin transporters have been associated with an increased risk of BPD and of mania in response to the administration of antidepressant medications [44]. The sphingosine pathway has not been extensively examined in terms of BPD. One GWAS study identified a BPD risk region located near the gene encoding sphingosine-1-phosphate receptor 1 (S1PR1) [45]. Based on our findings, additional studies of the role of sphingosine in mania and BPD may be warranted.
The specific biochemical mechanisms by which the ingested dietary nitrate can lead to altered behavior and gene expression in the brain are currently unclear but may include altered levels of biological mediators that are derived from dietary nitrate. One possible candidate is nitric oxide (NO), which can vary by level of dietary nitrate intake [46]. Dysregulation in the NO pathway has been implicated in BPD. Meta-analysis of oxidative stress markers reveal that patients with BPD exhibit significantly increased plasma NO compared to healthy controls [47]. NO also interacts with several physiological pathways, including those regulating serotonin [48], NF-κB [49], TLRs and other microbial pattern recognition molecules [50], and sphingosine-1 phosphate signaling [51]. These are the same pathways we found to be dysregulated in the brains of rats fed nitrated meat preparations and that are also potentially altered in individuals with BPD. The effect of nitrates on brain pathways found by microarray analysis should be confirmed by additional gene-expression- and protein-based methods in order to verify the associations and to identify additional pathways that might be dysregulated following exposure to dietary nitrates.
It is also possible that the behavioral effects of added nitrate are mediated by changes we measured in the intestinal microbiota (Fig. 4). This association is of interest in light of recent studies indicating altered microbiota in individuals with mania [52] as well as with other psychiatric disorders [53, 54]. The specific microbial taxa found to be increased in small intestinal samples from the rats with the diet containing added nitrate, Lachnospiraceae and Erysipelotrichales, are of particular interest since this family of microorganisms have been associated with altered behavior and cognitive functioning in experimental animals [55–57]. These organisms are also found in the human gastrointestinal tract where they are associated with altered fat and energy metabolism [58, 59]. It has been shown that the interaction between the gut microenvironment and its microbiome is bidirectional and influences central nervous system function both directly and indirectly through the gut–brain axis. This is particularly interesting in the context of brain NO signaling as discussed above. Following ingestion, a portion of dietary nitrate is converted to nitrite in the oral cavity; this influx of nitrite to the gut both increases its levels throughout the body and promotes its microbe-mediated reduction to NO in the gut [60]. It is plausible that the microbiome changes we see in the nitrate-fed rats may contribute to their mania-like brain and behavior dysregulation and may do so in part by mediating the effects of dietary nitrate.
In recent years, ingestion of cured meat has been implicated in a range of diseases, although the mechanisms involved in the disease associations remain largely undetermined [61]. To our knowledge, this is the first study associating exposure to cured meat with a neuropsychiatric disorder. As in the studies in other fields suggesting an association between cured meat consumption and pathophysiological effects, it is important to note potential confounds. Though we attempted to account for many of these confounds in our analysis, our analyses are limited by the retrospective and qualitative nature of our exposure survey. We thus modeled intake of both cured meat and dietary nitrate itself in animals, in which we could measure and control intake, control for potential confounds through our experimental design, and evaluate both behavioral and physiological outcomes following a defined period of exposure. The mania-like changes we see in both behavior and brain analysis of our rats calls for further investigation into a definitive mechanism that could further facilitate our understanding of human mania and add to our understanding of other pathophysiological correlates of nitrate exposure.
Conclusion
Our study indicates that exposure to nitrated meat products is increased in a cohort of individuals with mania. Furthermore, the feeding of preparations containing added nitrates to rats results in mania-like hyperactivity, alterations in hippocampal pathways implicated in human BPD, and changes to gut microbiota. While further investigations are warranted, individuals at risk for mania may consider limiting ingestion of added dietary nitrates.
Supplementary Material
Acknowledgements
This work was supported by a NIMH P50 Silvio O. Conte Center at Johns Hopkins (grant# MH-94268), the American Academy of Neurology Medical Student Research Award, the William C. Walker fund of the Johns Hopkins Department of Psychiatry and Behavioral Science, and by the Stanley Medical Research Institute.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41380-018-0105-6) contains supplementary material, which is available to authorized users.
References
- 1.Uher R. Gene-environment interactions in severe mental illness. Front Psychiatry. 2014;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes TM. Diet, inflammation and the brain: commentary on the 2014 named series. Brain Behav Immun. 2014;42:6–9. [DOI] [PubMed] [Google Scholar]
- 3.Jacka FN, Pasco JA, Mykletun A, Williams LJ, Nicholson GC, Kotowicz MA, et al. Diet quality in bipolar disorder in a population-based sample of women. J Affect Disord. 2011;129:332–7. [DOI] [PubMed] [Google Scholar]
- 4.Rios AC, Maurya PK, Pedrini M, Zeni-Graiff M, Asevedo E, Mansur RB, et al. Microbiota abnormalities and the therapeutic potential of probiotics in the treatment of mood disorders. Rev Neurosci. 2017;28:739–49. [DOI] [PubMed] [Google Scholar]
- 5.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Markers of gluten sensitivity in acute mania: a longitudinal study. Psychiatry Res. 2012;196:68–71. [DOI] [PubMed] [Google Scholar]
- 6.Saunders EF, Ramsden CE, Sherazy MS, Gelenberg AJ, Davis JM, Rapoport SI. Reconsidering dietary polyunsaturated fatty acids in bipolar disorder: a translational picture. J Clin Psychiatry. 2016;77:e1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Estecha M, Trasobares EM, Tajima K, Cano S, Fernandez C, Lopez JL, et al. Trace elements in bipolar disorder. J Trace Elem Med Biol. 2011;25(Suppl 1):S78–83. [DOI] [PubMed] [Google Scholar]
- 8.Anderson G, Maes M. Bipolar disorder: role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Curr Psychiatry Rep. 2015;17:8. [DOI] [PubMed] [Google Scholar]
- 9.Severance EG, Tveiten D, Lindstrom LH, Yolken RH, Reichelt KL. The gut microbiota and the emergence of autoimmunity: relevance to major psychiatric disorders. Curr Pharm Des. 2016;22:6076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–8. [DOI] [PubMed] [Google Scholar]
- 11.Severance EG, Lin J, Sampson HA, Gimenez G, Dickerson FB, Halling M, et al. Dietary antigens, epitope recognition, and immune complex formation in recent onset psychosis and long-term schizophrenia. Schizophr Res. 2011;126:43–50. [DOI] [PubMed] [Google Scholar]
- 12.Johnson IT. The cancer risk related to meat and meat products. Br Med Bull. 2017;121:73–81. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Rava M, Bedard A, Dumas O, Garcia-Aymerich J, Leynaert B, et al. Cured meat intake is associated with worsening asthma symptoms. Thorax. 2017;72:206–12. [DOI] [PubMed] [Google Scholar]
- 14.Dickerson F, Stallings C, Origoni A, Vaughan C, Katsafanas E, Khushalani S, et al. A combined marker of inflammation in individuals with mania. PLoS ONE. 2013;8:e73520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickerson F, Katsafanas E, Schweinfurth LA, Savage CL, Stallings C, Origoni A, et al. Immune alterations in acute bipolar depression. Acta Psychiatr Scand. 2015;132:204–10. [DOI] [PubMed] [Google Scholar]
- 16.Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60:466–72. [DOI] [PubMed] [Google Scholar]
- 17.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9. [DOI] [PubMed] [Google Scholar]
- 18.Mesuere B, Debyser G, Aerts M, Devreese B, Vandamme P, Dawyndt P. The Unipept metaproteomics analysis pipeline. Proteomics. 2015;15:1437–42. [DOI] [PubMed] [Google Scholar]
- 19.Dickerson F, Stallings C, Sullens A, Origoni A, Leister F, Krivogorsky B, et al. Association between cognitive functioning, exposure to Herpes Simplex Virus type 1, and the COMT Val158Met genetic polymorphism in adults without a psychiatric disorder. Brain Behav Immun. 2008;22:1103–7. [DOI] [PubMed] [Google Scholar]
- 20.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–9. [DOI] [PubMed] [Google Scholar]
- 21.Dickerson FB, Boronow JJ, Stallings CR, Origoni AE, Cole S, Yolken RH. Association between cognitive functioning and employment status of persons with bipolar disorder. Psychiatr Serv. 2004;55:54–8. [DOI] [PubMed] [Google Scholar]
- 22.Tsikas D, Fuchs I, Gutzki FM, Frolich JC. Measurement of nitrite and nitrate in plasma, serum and urine of humans by high-performance liquid chromatography, the Griess assay, chemiluminescence and gas chromatography-mass spectrometry: interferences by biogenic amines and N(G)-nitro-L-arginine analogs. J Chromatogr B Biomed Sci Appl. 1998;715:441–4. discussion 445–8. [PubMed] [Google Scholar]
- 23.Dailey MJ, Stingl KC, Moran TH. Disassociation between preprandial gut peptide release and food-anticipatory activity. Endocrinology. 2012;153:132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherrill JT, et al. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychol Med. 2000;30:1005–16. [DOI] [PubMed] [Google Scholar]
- 25.Sylvia LG, Alloy LB, Hafner JA, Gauger MC, Verdon K, Abramson LY. Life events and social rhythms in bipolar spectrum disorders: a prospective study. Behav Ther. 2009;40:131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curing and smoking meats for home food preservation literature review and critical preservation points. http://nchfp.uga.edu/publications/nchfp/lit_rev/cure_smoke_rev.html 2002.
- 27.Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meijer MK, Sommer R, Spruijt BM, van Zutphen LF, Baumans V. Influence of environmental enrichment and handling on the acute stress response in individually housed mice. Lab Anim. 2007;41:161–73. [DOI] [PubMed] [Google Scholar]
- 29.Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet. 2015;115:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker R. Nitrates, nitrites and N-nitrosocompounds: a review of the occurrence in food and diet and the toxicological implications. Food Addit Contam. 1990;7:717–68. [DOI] [PubMed] [Google Scholar]
- 31.Zhu S, Cordner ZA, Xiong J, Chiu CT, Artola A, Zuo Y, et al. Genetic disruption of ankyrin-G in adult mouse forebrain causes cortical synapse alteration and behavior reminiscent of bipolar disorder. Proc Natl Acad Sci USA. 2017;114:10479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164:1263–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott J, Murray G, Henry C, Morken G, Scott E, Angst J, et al. Activation in bipolar disorders: a systematic review. JAMA Psychiatry. 2017;74:189–96. [DOI] [PubMed] [Google Scholar]
- 34.Cheniaux E, Filgueiras A, Silva Rde A, Silveira LA, Nunes AL, Landeira-Fernandez J. Increased energy/activity, not mood changes, is the core feature of mania. J Affect Disord. 2014;152–4:256–61. [DOI] [PubMed] [Google Scholar]
- 35.Kirshenbaum GS, Clapcote SJ, Duffy S, Burgess CR, Petersen J, Jarowek KJ, et al. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase alpha3 sodium pump. Proc Natl Acad Sci USA. 2011;108:18144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abulseoud OA, Camsari UM, Ruby CL, Mohamed K, Abdel Gawad NM, Kasasbeh A, et al. Lateral hypothalamic kindling induces manic-like behavior in rats: a novel animal model. Int J Bipolar Disord. 2014;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowland MH, Hugunin KM, Rogers KL. Effects of short-term fasting in male Sprague-Dawley rats. Comp Med. 2011;61:138–44. [PMC free article] [PubMed] [Google Scholar]
- 38.Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neurosci Biobehav Rev. 2010;34:1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA. Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res. 2010;178:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logan RW, McClung CA. Animal models of bipolar mania: the past, present and future. Neuroscience. 2016;321:163–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira J, Busson M, Etain B, Jamain S, Hamdani N, Boukouaci W, et al. Polymorphism of Toll-like receptor 4 gene in bipolar disorder. J Affect Disord. 2014;152:395–402. [DOI] [PubMed] [Google Scholar]
- 42.Bueno BG, Caso JR, Madrigal JLM, Leza JC. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci Biobehav Rev. 2016;64:134–47. [DOI] [PubMed] [Google Scholar]
- 43.Elhaik E, Zandi P. Dysregulation of the NF-kappa B pathway as a potential inducer of bipolar disorder. J Psychiatr Res. 2015;70:18–27. [DOI] [PubMed] [Google Scholar]
- 44.Frye MA, McElroy SL, Prieto ML, Harper KL, Walker DL, Kung S, et al. Clinical risk factors and serotonin transporter gene variants associated with antidepressant-induced mania. J Clin Psychiatry. 2015;76:174–80. [DOI] [PubMed] [Google Scholar]
- 45.Xu W, Cohen-Woods S, Chen Q, Noor A, Knight J, Hosang G, et al. Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med Genet. 2014;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bondonno CP, Croft KD, Ward N, Considine MJ, Hodgson JM. Dietary flavonoids and nitrate: effects on nitric oxide and vascular function. Nutr Rev. 2015;73:216–35. [DOI] [PubMed] [Google Scholar]
- 47.Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–44. [DOI] [PubMed] [Google Scholar]
- 48.Moreira FA, Guimaraes FS. Role of serotonin receptors in panic-like behavior induced by nitric oxide in the rat dorsolateral periaqueductal gray: effects of chronic clomipramine treatment. Life Sci. 2005;77:1972–82. [DOI] [PubMed] [Google Scholar]
- 49.Ukil A, Biswas A, Das T, Das PK. 18 Beta-glycyrrhetinic acid triggers curative Th1 response and nitric oxide up-regulation in experimental visceral leishmaniasis associated with the activation of NF-kappa B. J Immunol. 2005;175:1161–9. [DOI] [PubMed] [Google Scholar]
- 50.Abdul-Cader MS, Amarasinghe A, Abdul-Careem MF. Activation of toll-like receptor signaling pathways leading to nitric oxidemediated antiviral responses. Arch Virol. 2016;161:2075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitz EI, Potteck H, Schuppel M, Manggau M, Wahydin E, Kleuser B. Sphingosine 1-phosphate protects primary human keratinocytes from apoptosis via nitric oxide formation through the receptor subtype S1P(3). Mol Cell Biochem. 2012;371:165–76. [DOI] [PubMed] [Google Scholar]
- 52.Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. 2017;87:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwarz E, Maukonen J, Hyytiainen T, Kieseppa T, Oresic M, Sabunciyan S, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2017;92:398–403. [DOI] [PubMed] [Google Scholar]
- 54.Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guida F, Turco F, Iannotta M, De Gregorio D, Palumbo I, Sarnelli G, et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun. 2017;67:230–45. [DOI] [PubMed] [Google Scholar]
- 56.Gacias M, Gaspari S, Santos PM, Tamburini S, Andrade M, Zhang F, et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife. 2016;5:e13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magnusson KR, Hauck L, Jeffrey BM, Elias V, Humphrey A, Nath R, et al. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience. 2015;300:128–40. [DOI] [PubMed] [Google Scholar]
- 58.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, et al. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes. 2016;65:2214–23. [DOI] [PubMed] [Google Scholar]
- 59.Cooper DN, Kable ME, Marco ML, De Leon A, Rust B, Baker JE, et al. The effects of moderate whole grain consumption on fasting glucose and lipids, gastrointestinal symptoms, and microbiota. Nutrients. 2017;9:E173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pereira C, Ferreira NR, Rocha BS, Barbosa RM, Laranjinha J. The redox interplay between nitrite and nitric oxide: from the gut to the brain. Redox Biol. 2013;1:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohrmann S, Linseisen J. Processed meat: the real villain? Proc Nutr Soc. 2016;75:233–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


