Summary
A growing body of evidence has indicated that the release of nociceptive factors, such as interleukins and chemokines, by activated immune and glial cells has crucial significance for neuropathic pain generation and maintenance. Moreover, changes in the production of nociceptive immune factors are associated with low opioid efficacy in the treatment of neuropathy. Recently, it has been suggested that CC chemokine receptor type 1 (CCR1) signaling is important for nociception. Our study provides evidence that the development of hypersensitivity in rats following chronic constriction injury (CCI) of the sciatic nerve is associated with significant up‐regulation of endogenous CCR1 ligands, namely, CCL2, CCL3, CCL4, CCL6, CCL7 and CCL9 in the spinal cord and CCL2, CCL6, CCL7 and CCL9 in dorsal root ganglia (DRG). We showed that single and repeated intrathecal administration of J113863 (an antagonist of CCR1) attenuated mechanical and thermal hypersensitivity. Moreover, repeated administration of a CCR1 antagonist enhanced the analgesic properties of morphine and buprenorphine after CCI. Simultaneously, repeated administration of J113863 reduced the protein levels of IBA‐1 in the spinal cord and MPO and CD4 in the DRG and, as a consequence, the level of pronociceptive factors, such as interleukin‐1β (IL‐1β), IL‐6 and IL‐18. The data obtained provide evidence that CCR1 blockade reduces hypersensitivity and increases opioid‐induced analgesia through the modulation of neuroimmune interactions.
Keywords: buprenorphine, CCR1, interleukins, J113863, morphine
Our results give evidence that CCR1 is a promising target for pain relief because J113863 is able to reduce hypersensitivity after chronic constriction injury of the sciatic nerve and simultaneously enhance opioid efficacy. After the nerve injury, J113863 prevents up‐regulation of pronociceptive IL‐1β, IL‐18 and IL‐6 by reducing the activity and infiltration of IBA‐1‐positive cells, neutrophils and CD4 lymphocytes and increases the level of antinociceptive IL‐1RA. This supports the hypothesis that the pharmacological modulation of neuroimmunological interactions via CCR1 may represent a new strategy for effective polytherapy with opioids in patients suffering from neuropathic pain.
Abbreviations
- CCI
chronic constriction injury
- CCL
CC motif chemokine ligand
- CCR
chemokine receptor type
- CXCR
CXC chemokine receptor type
- DOR
delta‐opioid receptor
- DRG
dorsal root ganglia
- GFAP
glial fibrillary acidic protein
- HPRT
hypoxanthine phosphoribosyltransferase
- IBA‐1
ionized calcium‐binding adapter molecule‐1
- IL
interleukin
- IL‐1RA
interleukin‐1 receptor antagonist
- i.p.
intraperitoneal
- i.t.
intrathecal
- MOR
mu‐opioid receptors
- MPO
myeloperoxidase
- NOS2
nitric oxide synthase 2, inducible
- RAG
recombinase activating genes
- RT‐qPCR
quantitative reverse transcription polymerase chain reaction
Introduction
Approximately 5–10% of the population worldwide suffers from neuropathic pain.1, 2 This pathological state is caused by a lesion or disease of the somatosensory nervous system, which can result from injury or a medical condition such as cancer, diabetes or autoimmune diseases.1, 3 The treatment of neuropathic pain is still challenging. Even strong analgesics such as opioids become inefficient.4, 5 Recent studies, including ours, have suggested that a disturbed balance between pronociceptive factors [e.g. interleukin‐1β (IL‐1β), IL‐18, IL‐6 and nitric oxide synthase 2 (NOS2)] and antinociceptive factors [e.g. IL‐1 receptor antagonist (IL‐1RA), IL‐18 binding protein (IL‐18BP) and IL‐10] contributes to the development of neuropathic pain.6, 7, 8, 9 Moreover, research on chemokines has suggested that their systems (ligands/receptors) are involved in pathological nociceptive processes.10, 11, 12 Our recent results indicated that the blockade of some chemokine receptors (e.g. CCR2 and CCR5) reduces symptoms of neuropathic pain in a chronic constriction injury (CCI) model and enhance the analgesic properties of opioids.13, 14, 15 These favorable results, as well as other reports in the literature, indicate the crucial role of CC family receptors in neuropathic pain development.
Although studies on CC group chemokine receptors have been conducted, there is still no information about the role of CC chemokine receptor type 1 (CCR1) in a CCI model of neuropathic pain. Hence, we investigated the role of this receptor in pathological nociception. This G‐protein‐coupled receptor from the β subfamily16 is present on the surface of neurons,17, 18 astrocytes,17, 18 microglia,7, 17, 18 neutrophils,19, 20 basophils,21 monocytes,19, 21 eosinophils,21 T cells19, 22 and immature dendritic cells.19, 21. CCR1 seems to be a very important receptor because it is a target of several chemokines [CC motif chemokine ligand 2 (CCL2), CCL3, CCL4, CCL5, CCL6, CCL7 and CCL9],4, 23, 24, 25, 26, 27 which have been suggested to be involved in the pathogenesis of neuropathic pain.28, 29, 30 There is some evidence demonstrating that CCR1 is involved in nociception. First, Kiguchi et al. 31 showed that the up‐regulation of CCR1/CCL3 is associated with the development of pain‐related symptoms in a model of murine partial sciatic nerve ligation. Second, Matsushita et al. 32 demonstrated that BX513 hydrochloride (an antagonist of CCR1) is able to inhibit recombinant CCL3‐induced mechanical hypersensitivity. Additionally, in a mouse model of diabetic neuropathic pain, strong spinal up‐regulation of two CCR1 ligands (CCL3 and CCL9) in strict association with the development of painful symptoms has been observed. Moreover, intraperitoneal (i.p.) injection of a CCR1 antagonist (J113863) as well as the neutralization of CCL3 and CCL9 reduces symptoms of diabetic neuropathic pain.28 Furthermore, our latest data demonstrated that neutralization of two CCR1 endogenous ligands (CCL2 and CCL7) strongly attenuated CCI‐induced neuropathic pain and significantly affected opioid effectiveness, highlighting the role of CCL2 and CCL7 in the pathogenesis of neuropathic pain.29 Because of the pleiotropic nature of the chemokines, their ability to activate many receptors, as well as the activation of CCR1 by many different chemokines, we wanted to confront previous results and assess which pharmacological manipulation (receptor blockade or ligand neutralization) may be more important in reducing symptoms of neuropathic pain and modulation of nociceptive factors. As presented in the paper by Kwiatkowski et al. 29 such a pharmacological approach is based on targeting a specific chemokine, whereas the idea of using an antagonist may give a broader effect. To our knowledge, there are two chemokine receptor antagonists successfully applied in the clinic, but not in neuropathic pain treatment. These are maraviroc (CCR5 antagonist), an antiretroviral drug used against the human immunodeficiency virus, and plerixafor (CXCR4 antagonist) used in the treatment of non‐Hodgkin lymphoma and multiple myeloma.33, 34 We believe that our tested antagonist of CCR1 may bring some beneficial properties in the treatment of neuropathic pain and will become a substance used in the clinic.
Due to the lack of information about the gene expression of endogenous ligands of CCR1 in neuropathic pain after nerve injury, we decided to investigate time‐dependent changes in their mRNA levels on the days 2, 7, 14 and 28 after CCI of the sciatic nerve in rats. Then, we investigated whether single or repeated intrathecal (i.t.) injections of the CCR1 antagonist J113863 influenced the hypersensitivity that developed and was maintained after CCI of the sciatic nerve. Furthermore, we tested whether J113863 enhanced the analgesic effects of morphine and buprenorphine. We studied whether and how repeated intrathecal administration of CCR1 antagonist influenced the level of IBA‐1 (a microglial/macrophage marker), glial fibrillary acidic protein (GFAP; an astrocyte/satellite cell marker), myeloperoxidase (MPO; a neutrophil marker), CD4 and CD8 (T lymphocyte markers) in the spinal cord and dorsal root ganglia (DRG) on the 7th day post‐CCI. In parallel, we decided to measure the level of factors with pronociceptive (IL‐1β, IL‐18 and IL‐6) and antinociceptive (IL‐10, IL‐1RA and IL‐18BP) properties in vehicle‐treated and J113863‐treated groups of rats.
Materials and methods
Animals
Male Wistar rats (275–300 g) were housed in cages covered with sawdust with food and water available ad libitum under a standard 12‐hr/12‐hr light/dark cycle (lights on at 08.00). The animals were allowed to acclimate to the environment for approximately 5 min before the behavioral tests. All experiments were performed according to the recommendations of the International Association for the Study of Pain35 and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Ethical Committee of the Maj Institute of Pharmacology of the Polish Academy of Sciences (LKE: 1277/2015). According to the 3R policy, the number of rats was reduced to the necessary minimum. Animal studies are reported in compliance with the ARRIVE guidelines.
Intrathecal injection
Catheters for i.t. injections were implanted according to the method described by Yaksh and Rudy.36 Before surgery, the rats were anesthetized i.p. with sodium pentobarbital (60 mg/kg). Polyethylene tubing catheters (13‐cm‐long, PE 10, intramedic; Clay Adams, Parsippany, NJ) with a dead space of 10 μl were sterilized by immersion in 70% (volume/volume) ethanol and fully washed out with water for injection immediately before surgery. During insertion, 7·8 cm of each catheter was slowly introduced through the atlanto‐occipital membrane into the subarachnoid space at the rostral level of the spinal cord lumbar enlargement (L4–L5). After surgery, water (10 μl) was injected, and the catheters were tightened. All rats recovered for 7 days before further procedures.
Chronic constriction injury
Chronic constriction injury of the sciatic nerve in rats was performed under sodium pentobarbital anesthesia (60 mg/kg, i.p.). According to the procedure described by Bennett and Xie,37 an incision was made below the hip bone, and the biceps femoris and gluteus superficialis were separated. The exposed right sciatic nerve was loosely tied four times with 1‐mm spacing using 4/0 silk ligatures until a brief twitch in the operated hind limb appeared. As a result of the limitation of procedures by ethical committees and our already obtained results we can only use CCI‐exposed rats in comparison with naive rats. In our previous study,38 we indicated that there were no significant differences in the nociceptive responses and levels of nociceptive factors between naive and sham‐operated animals, for example on day 7, the cold‐plate test values were as follows: naive 28·0 ± 0·8 s and sham 28·5 ± 0·9 s; for biochemical studies of dorsal lumbar spinal cord, e.g. for IL‐18, IL‐6 there were also no differences between naive and sham‐operated groups 1 ± 0·2 versus 0·9 ± 0·2; 1 ± 0·1 versus 1·1 ± 0·1, respectively.
Drug administration
In the experiment, we used the following substances: J113863 (J11) (1,4‐cis‐1‐(1‐cycloocten‐1‐ylmethyl)‐4‐[[(2,7‐dichloro‐9H‐xanthen‐9‐yl)carbonyl]amino]‐1‐ethylpiperidinium iodide, Tocris, Bristol, UK, https://www.tocris.com/products/j-113863_2595), morphine hydrochloride (M; TEVA, Kutno, Poland) and buprenorphine (B; Polfa Warszawa SA, Warsaw, Poland). J113863 was dissolved in 0·5% dimethyl sulfoxide (DMSO) in water for injection, whereas morphine and buprenorphine were dissolved in water for injection. The first group of animals received J113863 pre‐emptively 16 and 1 hr before CCI surgery at a dose of 0·1 μg/5 μl (i.t.) and then once a day for the following 7 days. The control rats received vehicle (V) (0·5% DMSO in water for injection) according to the same schedule. The behavioral tests (von Frey and cold‐plate tests) were performed on the 2nd and 7th days post‐CCI 3 hr after J113863 or vehicle injection (Fig. 3a). Additionally, on the 6th day after CCI surgery, some of the chronically treated rats received a single dose of morphine (2·5 μg/5 μl, i.t.) or buprenorphine (1·0 μg/5 μl, i.t.) 30 min after J113863 administration. For these animals, the same behavioral tests were conducted 30–35 min after opioid injection (Fig 7a). Another group of animals received a single injection of J113863 (0·1 μg/5 μl) or vehicle (0·5% DMSO in water for injection) on the 12th day post‐CCI, when fully developed thermal and mechanical hypersensitivity was observed. Behavioral reactions were measured using the same tests as those used previously. In addition, we carried out both tests in rats on the 2nd, 7th, 14th and 28th days after sciatic nerve surgery in the absence of drug injection to evaluate time‐dependent changes in mechanical and thermal hypersensitivity. The tests were always performed in the same order (first the von Frey test and then the cold‐plate test). The J113863 (0·1 μg/5 μl) dose was selected on the basis of our preliminary studies; the three doses – 0·1, 0·3 and 1 μg/5 μl – were tested. The doses 0·1 and 0·3 μg/5 μl produced significant dose‐dependent analgesic effects after their single administration. The dose 0·3 μg/5 μl produced very strong analgesia (V‐ versus J11‐treated: 12·88 ± 2·17 g versus 22·81 ± 3·40 g for von Frey test and 10·52 ± 1·56 s versus 29·5 ± 1·0 s for cold‐plate test). The dose 1 μg/5 μl evoked some side effects, like slight convulsions. Therefore, for repeated administration, we have chosen the lowest effective dose that does not cause side effects.
Figure 3.
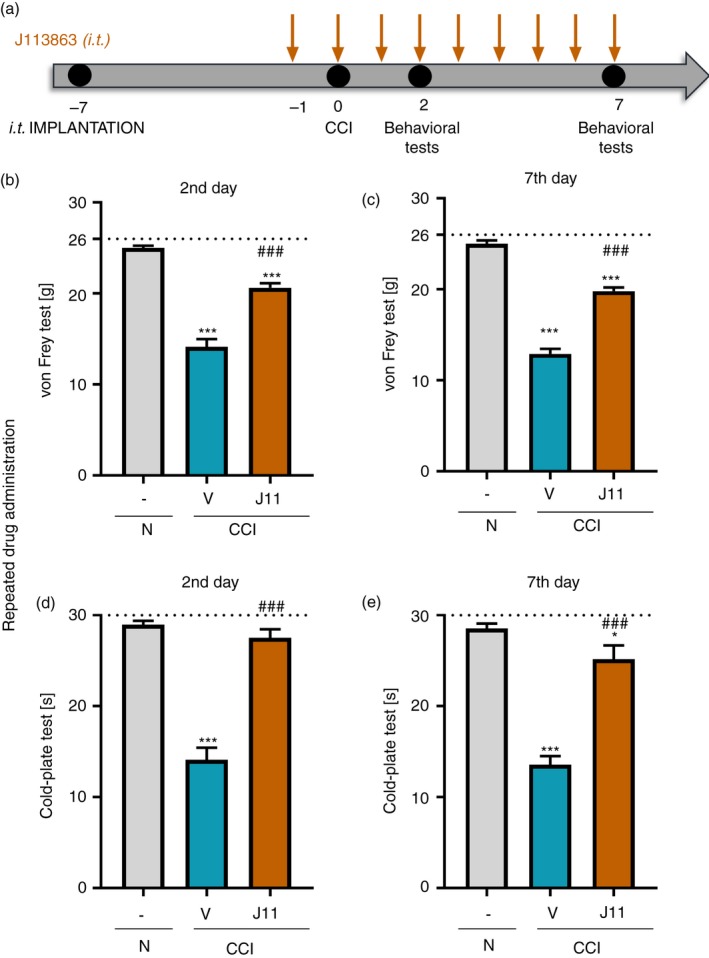
Effects of repeated administration of J113863 (0·1 μg/5 μl; intrathecally a) 3 hr after drug injection on pain‐related behaviors in rats on the 2nd and 7th days post‐CCI (b, c, von Frey test; d, e cold plate test). The horizontal dotted line shows the cut‐off value. The data are presented as the means ± SEM (15 rats per group). Intergroup differences were analyzed using analysis of variance with Bonferroni's multiple comparisons post hoc test. *P < 0·05 and ***P < 0·001 indicate a significant difference compared with the control group (naive animals); ### P < 0·001 indicates a significant difference compared with the vehicle‐treated group. Abbreviations: CCI, chronic constriction injury; J11, J113863; N, naive; V, vehicle.
Figure 7.
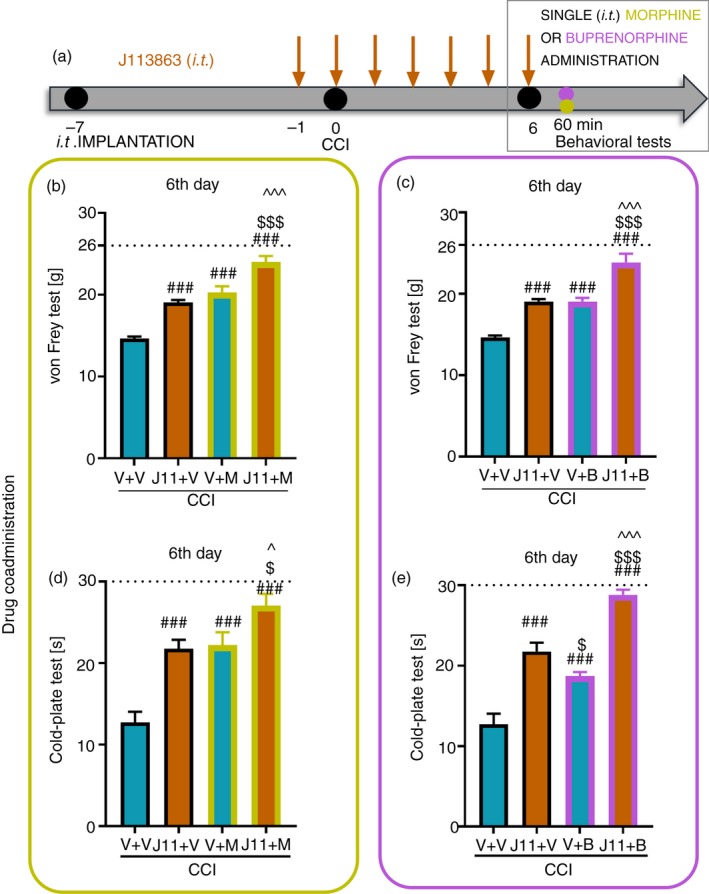
Effect of repeated administration of J113863 (0·1 μg/5 μl; intrathecally, a) 16 and 1 hr before CCI and then once a day for 6 days on pain‐related behaviors in rats (b, c von Frey test; d, e cold‐plate test) and the analgesic effects of morphine (b, d) (2·5 μg/5 μl, intrathecally) and buprenorphine (c, e) (1·0 μg/5 μl, intrathecally) 1 hr after J113863 or vehicle injections on the 6th day post‐CCI,. The data are presented as the mean ± SEM of 7–8 rats per group. Intergroup differences were analyzed using analysis of variance with Bonferroni's multiple comparisons post hoc test. ### P < 0·001 indicates a significant difference compared with the vehicle‐treated group; $ P < 0·05 and $$$ P < 0·001 indicate a significant difference compared with the J113863 + V‐treated group; ^P < 0·05 and ^^^P < 0·001 indicate a significant difference between the V + M‐ or V + B‐treated rats and J113863 + M‐ or J113863 + B‐treated rats. Abbreviations: B, buprenorphine; CCI, chronic constriction injury, J11, J113863; M, morphine; V, vehicle.
Mechanical hypersensitivity (von Frey test)
The von Frey apparatus (Dynamic Plantar Anesthesiometer, Cat. No. 37400, Ugo Basile, Italy) was used to measure mechanical hypersensitivity in rats, as previously described.14, 39 The CCI‐exposed animals were placed in plastic cages with a wire mesh floor 5 min before the experiment. The rats were able to move freely on the surface. The machine's touch stimulator was moved under the operated hind limb, and the reaction of the animal to the stimulus was measured automatically. The strength of the von Frey touch stimulator was up to 26 g.
Thermal hypersensitivity (cold‐plate test)
Thermal hypersensitivity was measured using a cold‐plate apparatus (Cold/Hot Plate Analgesia Meter No. 05044 Columbus Instruments, Columbus, OH), as previously described.39, 40 Each animal was placed on a cold surface (5°) and kept there until it lifted its hind paw. The cut‐off latency was 30 s.
Analysis of gene expression (RT‐qPCR)
To analyze the time‐dependent changes in the levels of chemokines, the dorsal lumbar segments of the spinal cord (L4–L6) and the DRG (L4–L6) were collected immediately from CCI rats after decapitation on the 2nd, 7th, 14th and 28th days post‐CCI and from naive animals. According to the method described by Chomczynski and Sacchi,41 total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). To measure the concentration of RNA in a single sample, a DeNovix DS‐11 Spectrophotometer (DeNovix Inc., Wilmington, NC) was used. Reverse transcription was performed on 1 μg of total RNA from the tissue at 37° for 1 hr using Omniscript reverse transcriptase (Qiagen Inc., Hilden, Germany). The reaction was performed in the presence of an RNAse inhibitor (rRNasin; Promega, Mannheim, Germany) and oligo(dT16) primers (Qiagen Inc.). The resulting cDNA templates were diluted 1:10 with water, and approximately 50 ng of cDNA from each animal was used for quantitative real‐time reverse transcription polymerase chain reaction (RT‐qPCR). RT‐qPCR was performed using Assay‐On‐Demand TaqMan probes (Applied Biosystems, Foster City, CA) on an iCycler device (Bio‐Rad, Warsaw, Poland) according to the manufacturer's protocols. The following TaqMan primers and probes were used: CCL2 (Rn00580555_m1), CCL3 (Rn01464736_g1), CCL4 (Rn00671924_m1), CCL5 (Rn00579590), CCL6 (Rn01456400_m1), CCL7 (Rn01467286_m1), CCL9 (Rn01471276_m1) and HPRT (Rn01527840_m1). A standard dilution curve was used to determine the amplification efficiency for each assay (between 1·7 and 2·0). The cycle threshold values were automatically calculated by cfxmanager v.2.1 software using the default parameters. The RNA content was calculated using the formula 2−(threshold cycle). The transcript level of HPRT (a housekeeping gene) was not significantly altered in the CCI‐exposed rats42 and therefore served as an adequate control. The RT‐qPCR method was described in our previous studies.13, 40
Analysis of protein levels (Western blot analysis)
Tissue from the dorsal lumbar segments of the spinal cord (L4–L6) and the DRG (L4–L6) was collected 6 hr after the last J113863/vehicle administration on the 7th day. Next, the samples were homogenized in RIPA buffer containing a protease inhibitor cocktail (Sigma‐Aldrich, St. Louis, MO) and cleared via centrifugation (30 min, 21952 G‐force, 4°). Total protein concentrations were measured using the bicinchoninic acid method. Samples (20 μg of protein) were heated in loading buffer (4× Laemmli buffer, Bio‐Rad) for 5 min at 98°. Electrophoresis was performed on 4–15% or 4–20% Criterion TGX precast polyacrylamide gels (Bio‐Rad). The proteins from the gels were transferred (semi‐dry transfer, 30 min, 25 V) to Immun‐Blot PVDF membranes (Bio‐Rad). Next, the membranes were blocked for 1 hr at room temperature using 5% non‐fat dry milk (Bio‐Rad) in Tris‐buffered saline with 0·1% Tween‐20 (TBST). Then, the membranes were washed in TBST buffer and incubated overnight at 4° with the following primary antibodies, rabbit: anti‐IBA‐1 (1:250; Proteintech, Manchester, UK), anti‐GFAP (1:10 000; Novus, Abingdon, UK), anti‐CD8 (1:500; Santa Cruz, Dallas, TX), anti‐MPO (1:1000; Abcam, Cambridge, UK), anti‐IL‐1β (1:500; Abcam), anti‐IL‐1RA (1:2000; Abcam), anti‐IL‐18 (1:1000; Abcam), anti‐IL‐18BP (1:500; Novus), anti‐IL‐6 (1:500; Invitrogen, Carlsbad, CA), anti‐IL‐10 (1:1000; Abcam); mouse: anti‐CD4 (1:1000; R&D Systems, Minneapolis, MN) and anti‐GAPDH (1:5000; Millipore, Darmstadt, Germany). Then, the membranes were washed in TBST buffer and incubated for 1 hr at room temperature in horseradish peroxidase‐conjugated anti‐rabbit or anti‐mouse secondary antibodies (Vector Laboratories, Burlingame, CA) at a dilution of 1:5000. To dilute the primary and secondary antibodies, SignalBoost™ Immunoreaction Enhancer Kit (Millipore) solution was used. Detection of selected proteins was performed using Clarity™ Western ECL Substrate (Bio‐Rad) and visualized on the Fujifilm LAS‐4000 FluorImager system. Fujifilm multi gauge software was used to estimate the levels of immunoreactive bands. All the bands are represented in a molecular weight (g/mol). The Western blot technique was described in our previous studies.15, 40
Statistical analysis
All data were analyzed using graphpad prism 8 software (GraphPad, San Diego, CA).
Behavioral analysis
For studies of time‐dependent changes in pain‐related behaviors in rats, the behavioral data are presented as the mean ± SEM in grams or seconds. The intergroup differences were analyzed using one‐way analysis of variance (anova) followed by Bonferroni's post hoc test for multiple comparisons. ***P < 0·001 indicates a significant difference compared with the control group (naive animals). For studies of single and repeated administration of J113863 or vehicle, the behavioral data are presented as the mean ± SEM in grams or seconds. The intergroup differences were analyzed using one‐way anova followed by Bonferroni's post hoc test for multiple comparisons. *P < 0·05 ***P < 0·001 indicates a significant difference compared with the control group (naive animals); ### P < 0·001 indicates a significant difference between the vehicle‐ and J113863‐treated groups. For studies of co‐administration of morphine or buprenorphine, the behavioral data are presented as the mean ± SEM in grams or seconds. The intergroup differences were analyzed using anova followed by Bonferroni's post hoc test for multiple comparisons. ### P < 0·001 indicates a significant difference compared with the vehicle‐treated group (V + V). $ P < 0·05, $$$ P < 0·001 indicates a significant difference compared with the J11 + V‐treated group. ^P < 0·05, ^^^P < 0·001 indicates a significant difference compared with V + M‐ or V + B‐treated rats and J11 + M or J11 + B‐treated rats.
RT‐qPCR analysis
The results are presented as the fold change in expression relative to the naive (control) group. The data are presented as the mean ± SEM and show the normalized averages derived from analyses of samples from each group. Differences between groups were analyzed using one‐way anova and Bonferroni's multiple comparisons post hoc test. *P < 0·05, **P < 0·01, and ***P < 0·001 indicate a significant difference compared with the control group (naive animals).
Western blot analysis
The data are presented as the fold change relative to the control (naive) group and are the normalized averages derived from analyses of samples from each group performed with the multi gauge analysis program. The intergroup differences were analyzed using anova followed by Bonferroni's post hoc test for multiple comparisons. *P < 0·05, **P < 0·01, and ***P < 0·001 indicate a significant difference compared with the control group (naive animals); # P < 0·05 and ## P < 0·01 indicate a significant difference compared with the vehicle‐treated group.
Results
Time‐dependent changes in the mRNA levels of CCL2, CCL3, CCL4, CCL5, CCL6, CCL7 and CCL9 in the spinal cord and DRG in parallel with pain‐related behaviors on the 2nd, 7th, 14th and 28th days post‐CCI
We observed strong mechanical (F = 49·89, P < 0·0001; Fig. 1a) and thermal (F = 148·8, P < 0·001; Fig. 1b) hypersensitivity on the 2nd day, as well as the other examined time‐points, post‐CCI. In our opinion, this hypersensitivity was associated with the up‐regulation of some ligands of CCR1. We noticed a significantly higher spinal level of CCL2 on the 2nd day (F = 8·153, P = 0·001; Fig. 1c) that persisted until the last day of the experiment, whereas in the DRG, a strong enhancement of the expression of CCL2 was observed only until the 7th day (F = 37·60, P < 0·0001) (Fig. 1j) after CCI. The level of CCL3 in the spinal cord was up‐regulated starting from the 7th day (F = 3·012, P = 0·0298) and persisting until the last day post‐CCI (Fig. 1d), and there were no significant changes in its level in the DRG (Fig. 1k). Similarly, we observed up‐regulated spinal levels of CCL4 beginning on the 2nd day (F = 4·503, P = 0·0047) and persisting until the last day of the experiment (Fig. 1e), but no changes in the DRG level were observed (Fig. 1l). CCL5 expression in the spinal cord was unchanged (Fig. 1f) after CCI; however, in the DRG, we observed a significant down‐regulation of its level on the 2nd day (F = 2·719, P = 0·0469) (Fig. 1m) as well as the 7th and 28th days. After CCI, CCL6 expression was up‐regulated starting from the 7th day and persisting until the last day of testing, both in the spinal cord (F = 4·206, P = 0·0069) and the DRG (F = 12·70, P < 0·0001) (Fig. 1g,n respectively). The most up‐regulated chemokine was CCL7. Its level was significantly higher on the 2nd day and at two other time‐points (7th and 28th) post‐CCI in the spinal cord (F = 26·88, P < 0·0001; Fig. 1h) and at all the examined time‐points until the 28th day in the DRG (F = 3·753, P = 0·0133; Fig. 1o). Down‐regulation of CCL9 was observed on the 2nd day in the spinal cord (F = 9·007, P < 0·0001; Fig. 1i), whereas in the DRG, the level of CCL9 was up‐regulated on the same day (F = 2·986, P = 0·0366; Fig. 1p). Additionally, there was an increase in the CCL9 level on the 14th day in both examined regions (Fig. 1i,p).
Figure 1.
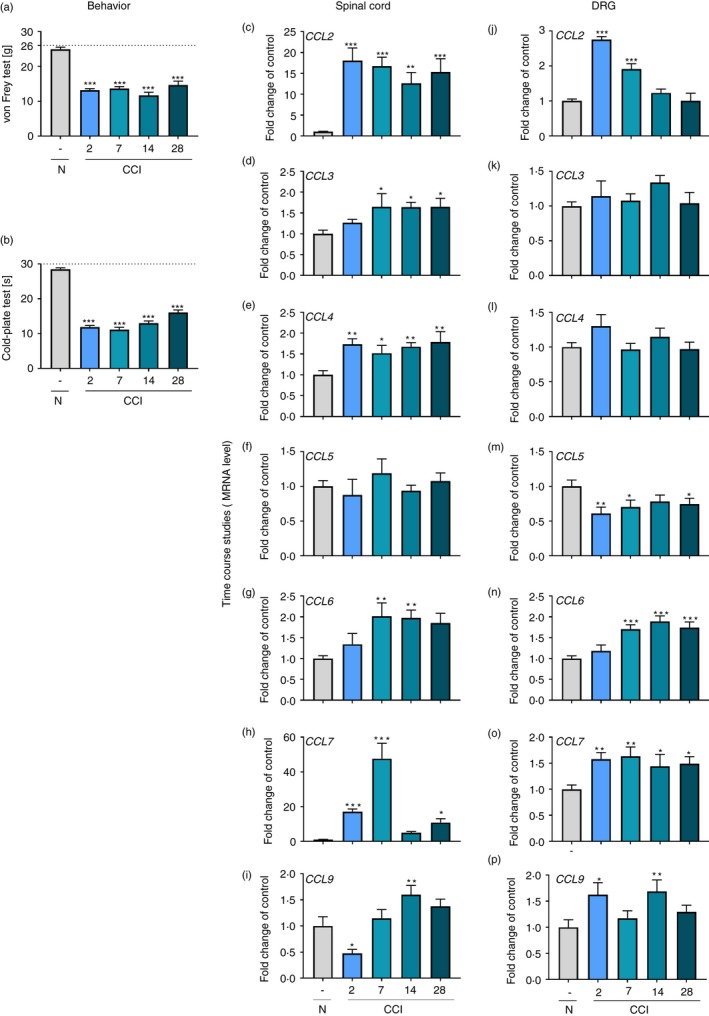
Time‐dependent changes in pain‐related behaviors in rats on the 2nd, 7th, 14th and 28th days after CCI (a, von Frey test; b, cold‐plate test) and associated changes in CCL2, CCL3, CCL4, CCL5, CCL6, CCL7, and CCL9 mRNA levels in the spinal cord (c–i) and in the dorsal root ganglion (DRG) (j–p). The behavioral data are presented as the means ± SEM of 10 rats per group. The RT‐qPCR data are presented as the means ± SEM of 5–10 samples per group. Intergroup differences were analyzed using analysis of variance with Bonferroni's multiple comparisons post hoc test. *P < 0·05, **P < 0·01, and ***P < 0·001 indicate a significant difference compared with the control group (naive rats). Abbreviations: CCI, chronic constriction injury; N, naive.
The effect of single i.t. administration of J113863 on pain‐related behaviors on the 12th day post‐CCI
Single i.t. injection of J113863 or vehicle was performed on the 12th day post‐CCI. Next, the von Frey (Fig. 2a) and cold‐plate (Fig. 2b) tests were performed. We observed that 12 days after CCI, animals developed strong mechanical hypersensitivity in the ipsilateral paw and responded to stimulation at a lower level than naive animals. J113863 attenuated mechanical hypersensitivity, and the animals had an elevated pain threshold (F = 70·53, P < 0·0001; Fig. 2a). Similar effects were observed in the cold‐plate test, in which the time for J113863‐treated rats to react to low temperatures was increased compared with that for vehicle‐treated animals (F = 33·57, P < 0·0001; Fig. 2b).
Figure 2.
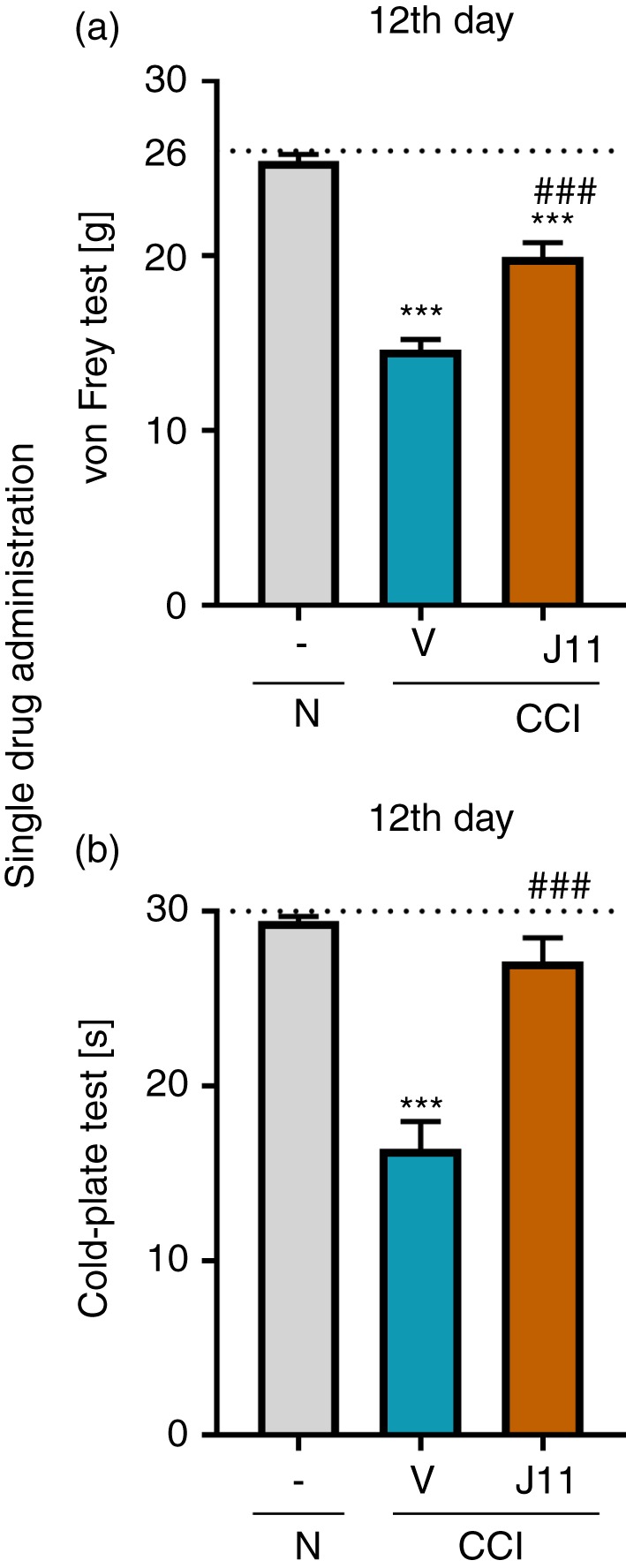
Effects of single administration of J113863 (0·1 μg/5 μl; intrathecally) 3 hr after drug injection on pain‐related behaviors in rats on the 12th day post‐CCI (a, von Frey test; b, cold‐plate test). The horizontal dotted line shows the cut‐off value. The data are presented as the means ± SEM (8–9 rats per group). Intergroup differences were analyzed using analysis of variance with Bonferroni's multiple comparisons post hoc test. ***P < 0·001 indicates a significant difference compared with the control group (naive animals); ### P < 0·001 indicates a significant difference compared with the vehicle‐treated group. Abbreviations: CCI, chronic constriction injury; J11, J113863; N, naive; V, vehicle.
The effect of repeated i.t. administration of J113863 on pain‐related behaviors on the 2nd and 7th days post‐CCI
Repeated administration of J113863 (Fig. 3a) significantly diminished mechanical and thermal hypersensitivity, as measured on the 2nd and 7th days post‐CCI. In the von Frey test on the 2nd (Fig. 3b) and 7th (Fig. 3c) days, the force at which J113863‐treated animals responded to stimulation with the filaments was higher than that of vehicle‐treated rats (F = 87·10, P < 0·0001 for the 2nd day and F = 179·4, P < 0·0001 for the 7th day). In the cold‐plate test on the 2nd (F = 73·34, P < 0·0001) (Fig. 3d) and 7th days (F = 53·70, P < 0·0001) (Fig. 3e), J113863 extended the pain response time in comparison with that of vehicle‐treated animals.
The effect of repeated i.t. administration of J113863 on the protein levels of IBA‐1, GFAP, CD4, CD8 and MPO in the spinal cord and the DRG on the 7th day post‐CCI
Chronic constriction injury induced a significant increase in the protein levels of IBA‐1 (F = 7·701, P = 0·0036 for the spinal cord and F = 30·88, P < 0·0001 for the DRG; Fig. 4a,f) and GFAP (F = 21·05, P < 0·0001 for the spinal cord and F = 11·60, P = 0·0005 for the DRG) (Fig. 4b,g). J113863 treatment diminished the spinal level of IBA‐1, whereas the GFAP protein level remained unchanged. In the DRG, after chronic treatment with the CCR1 antagonist, there were no changes in the level of IBA‐1 (Fig. 4f) or GFAP (Fig. 4g). Additionally, after CCI surgery, no changes in CD4 in the spinal cord were observed (Fig. 4c), but interestingly, the protein level was up‐regulated in the DRG (F = 7·592, P = 0·0033) J113863 significantly reduced the CD4 level in the DRG in comparison with that in vehicle‐treated animals (Fig. 4h). The protein level of CD8 did not change in either the spinal cord (Fig. 4d) or the DRG (Fig. 4i). In vehicle‐treated rats compared with naive animals, the MPO protein level was strongly increased in the spinal cord (F = 13·77, P = 0·0003; Fig. 4e) and DRG (F = 15·39, P = 0·0002; Fig. 4j). J113863 diminished the level of MPO in the DRG, whereas in the spinal cord, no changes were observed after chronic antagonist administration.
Figure 4.
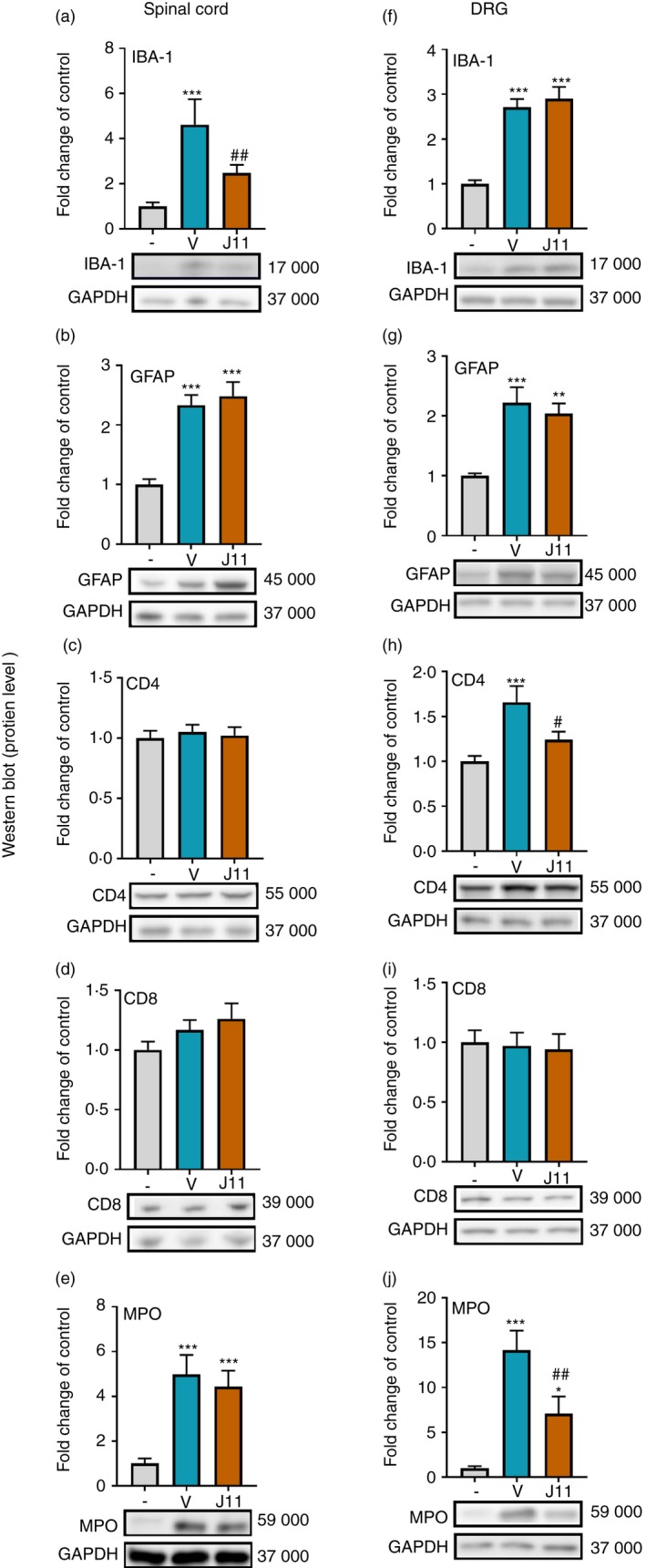
Effects of repeated administration of J113863 (J11; 0·1 μg/5 μl; intrathecally) 16 and 1 hr before CCI and then once a day for 7 days on the protein levels of IBA‐1 (a, f), GFAP (b, g), CD4 (c, h), CD8 (d, i) and MPO (e, j) in the spinal cord (a–e) and the dorsal root ganglion (DRG) (f–j) on the 7th day post‐CCI in rats. The data are presented as the mean fold change relative to the control ± SEM (6–8 samples per group). Intergroup differences were analyzed using analysis of variance with Bonferroni's multiple comparisons post hoc test. *P < 0·05, **P < 0·01 and ***P < 0·001 indicate a significant difference compared with the control group (naive animals); # P < 0·05 and ## P < 0·01 indicate a significant difference compared with the vehicle‐treated group. Abbreviations: CCI, chronic constriction injury; J11, J113863; V, vehicle.
The effect of repeated i.t. administration of J113863 on the protein levels of IL‐1β, IL‐1RA, IL‐18 and IL‐18BP in the spinal cord and the DRG on the 7th day post‐CCI
We did not observe any changes in the IL‐1β protein level in the spinal cord (Fig. 5a); however, in the DRG (Fig. 5e), the IL‐1β level was elevated after CCI (F = 5·124, P = 0·0154), and J113863 significantly diminished it. Moreover, J113863 increased the level of IL‐1RA in the spinal cord (Fig. 5b) and the DRG (Fig. 5f) compared with that in naive rats; however, there were no significant differences between vehicle‐treated and J113863‐treated animals. Moreover, in the spinal cord (Fig. 5c) and the DRG (Fig. 5g), we observed an increase in the level of IL‐18 in vehicle‐treated animals compared with naive rats (F = 12·97, P = 0·0002 for the spinal cord and F = 8·185, P = 0·0024 for the DRG). J113863 significantly attenuated these changes in the spinal cord but not in the DRG. There were no changes in IL‐18BP in either examined structure after CCI (Fig. 5d,h), and the CCR1 antagonist did not affect this level.
Figure 5.
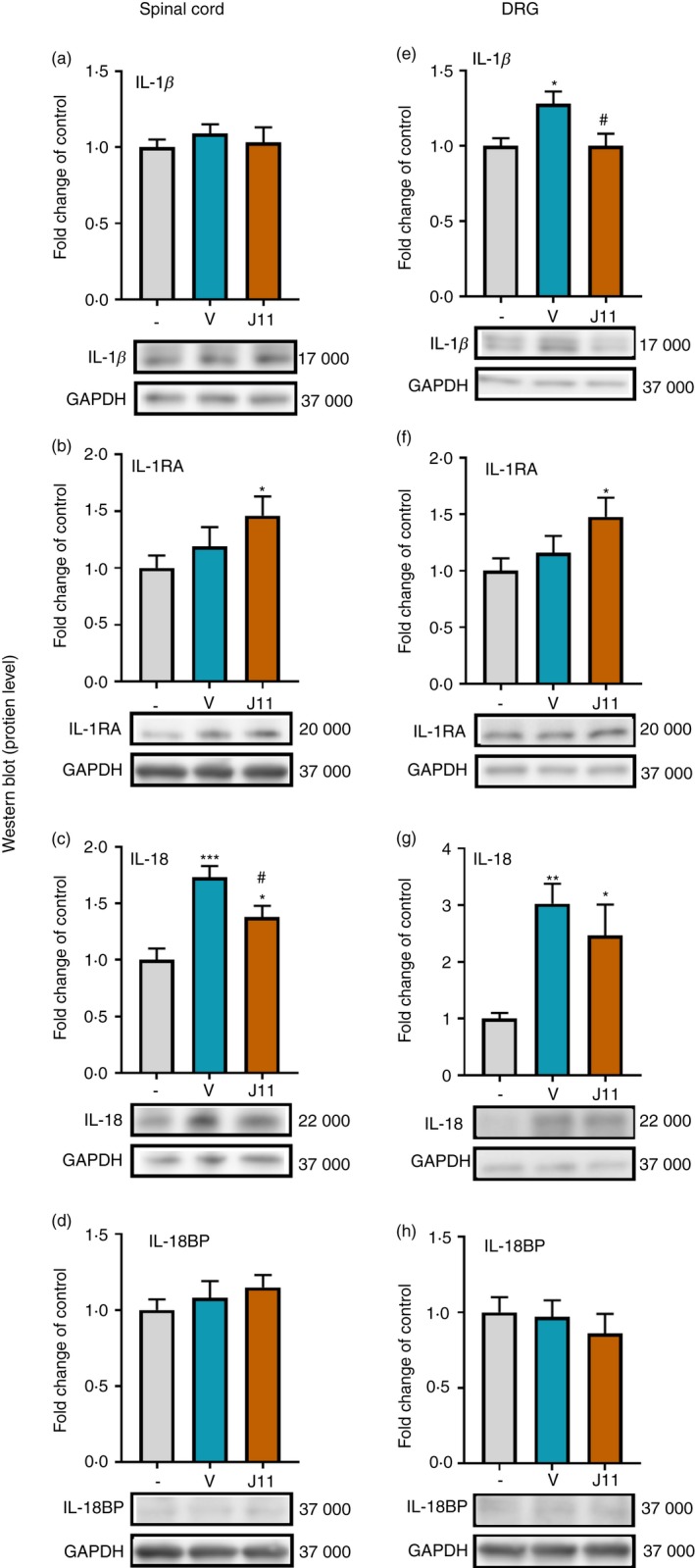
Effects of repeated administration of J113863 (0·1 μg/5 μl; intrathecally) 16 and 1 hr before CCI and then once a day for 7 days on the protein levels of IL‐1β (a, e), IL‐1RA (b, f), IL‐18 (c, g) and IL‐18BP (d, h) in the spinal cord (a–d) and the dorsal root ganglion (DRG) (e–h) on the 7th day post‐CCI in rats. The data are presented as the mean fold change relative to the control ± SEM (7–8 samples per group). Intergroup differences were analyzed using analysis of variance with Bonferroni's multiple comparisons post hoc test. *P < 0·05, **P < 0·01 and ***P < 0·001 indicate a significant difference compared with the control group (naive animals); # P < 0·05 indicates a significant differences compared with the vehicle‐treated group. Abbreviations: CCI, chronic constriction injury; IL‐1β, interleukin‐1β; J11, J113863; V, vehicle.
The effect of repeated i.t. administration of J113863 on the protein levels of IL‐6 and IL‐10 in the spinal cord and the DRG on the 7th day post‐CCI
There were no significant changes in the IL‐6 (Fig. 6a) and IL‐10 (Fig. 6b) protein levels in the spinal cord after CCI. Furthermore, no changes were observed in the level of IL‐10 in the DRG (Fig. 6d). CCI increased the protein level of IL‐6 in the DRG (F = 9·092, P = 0·0017; Fig. 6c), and J113863 significantly diminished it.
Figure 6.
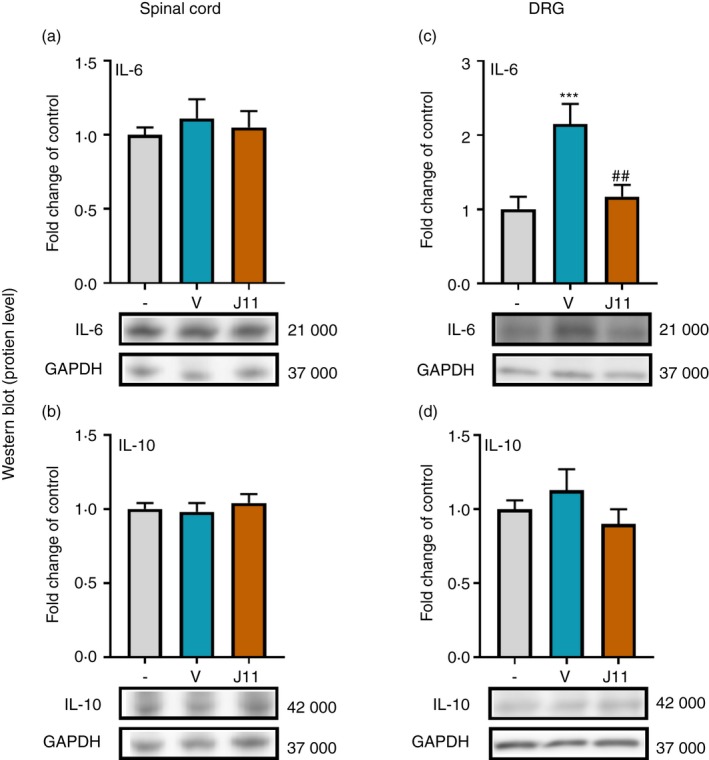
Effects of repeated administration of J113863 (0·1 μg/5 μl; intrathecally) 16 and 1 hr before CCI and then once a day for 7 days on the protein levels of interleukin‐6 (IL‐6) (a, c) and IL‐10 (b, d) in the spinal cord (a, b) and the dorsal root ganglion (DRG) (c, d) on the 7th day post‐CCI in rats. The data are presented as the mean fold change relative to the control ± SEM (7–8 samples per group). Intergroup differences were analyzed using analysis of variance with Bonferroni's multiple comparisons post hoc test. ***P < 0·001 indicates a significant difference compared with the control group (naive animals); ## P < 0·001 indicates a significant difference compared with the vehicle‐treated group. Abbreviations: CCI, chronic constriction injury; J11, J113863; V, vehicle.
The effect of repeated i.t. administration of J113863 on the analgesic effectiveness of morphine and buprenorphine on the 6th day post‐CCI
Behavioral tests (the von Frey test (Fig. 7b,c) and cold‐plate test (Fig. 7d,e)) were performed on the 6th day after repeated i.t. J113863 administration combined with a single i.t. injection of morphine (Fig. 7b,d) or buprenorphine (Fig. 7c,e), as shown in the diagram (Fig. 7a). Repeated J113863 administration reduced pain‐like behaviors in both tests. Moreover, both opioids reduced pain compared with vehicle. The effectiveness of morphine and/or buprenorphine was enhanced in the animals pretreated with J113863 in the von Frey test (F = 44·76, P < 0·0001 for morphine and F = 40·47, P < 0·0001 for buprenorphine) and cold‐plate test (F = 20·14, P < 0·0001 for morphine and F = 46·80, P < 0·0001 for buprenorphine).
Discussion
To our knowledge, the current study is the first attempt to determine the time‐dependent changes in the levels of endogenous ligands of CCR1 in rats. In the initial stage of neuropathic pain development, we observed particularly high levels of CCL2, CCL4, CCL7 and CCL9, which suggests their role in the development of hypersensitivity. Our data also indicated that CCL2, CCL3, CCL4, CCL6 and CCL7 seem to be important for the maintenance of neuropathic pain because we observed that their levels were up‐regulated until the 28th day post‐CCI. Moreover, we proved that single and repeated administration of the CCR1 antagonist J113863 attenuated CCI‐induced mechanical and thermal hypersensitivity. Importantly, our investigation showed that co‐administration of J113863 with morphine and buprenorphine enhanced its analgesic effect. Our results provide evidence that repeatedly administered CCR1 antagonist is able to relieve pain by reducing the activation and infiltration of microglia, macrophages, neutrophils and lymphocytes into the central and/or peripheral nervous system and hence by inducing beneficial changes in the levels of factors with pronociceptive (IL‐1β, IL‐6 and IL‐18) and antinociceptive (IL‐1RA) properties. The modulation of CCR1 seems to be an important target for novel potential therapies of neuropathic pain; however, future studies need to be performed to confirm this assertion.
It has been suggested that CCR1 is involved in the pathogenesis of diseases with large neuroimmunological components, such as rheumatoid arthritis and multiple sclerosis.19, 43 Our current results indicate that CCR1 is also essential for the development of neuropathic pain. We have proven that J113863 can not only induce analgesia after repeated administration but also attenuate fully developed hypersensitivity after a single injection. Our results are in agreement with research by Rojewska et al.,28 which demonstrated that a single intrathecal injection of J113863 decreases pain‐like behavior in a murine model of painful diabetic neuropathy. There is also evidence that J113863 decreases cancer pain caused by the intratibial inoculation of fibrosarcoma cells.44 Moreover, Lewis et al. 45 proved that the blockade of CCR1 using BI64 contributes to a reduction in mechanical hypersensitivity in a rat model of inflammatory pain. All of these facts, together with our present results, indicate that CCR1 is a promising target for pain treatment.
CCR1 appears to be important for pain processing, as many CC chemokines that act via this receptor have pronociceptive properties. It has been shown that injection of CCL2, CCL3, CCL4, CCL7 and CCL9 may lead to the development of pain‐like behavior in naive animals.28, 29, 30 Here, we demonstrated that chronic constriction injury led to an increase in the mRNA levels of the majority of CCR1 ligands at different time‐points. On the 2nd day post‐CCI, we observed increased levels of CCL2, CCL4, CCL7 and CCL9 in the spinal cord and/or the DRG. Based on the obtained results, we suggest that these chemokines play important roles in the first stage of neuropathic pain development. Among these cytokines, CCL2, CCL4 and CCL7, like CCL3 and CCL6, seem to also be important for the maintenance of neuropathy, and higher levels were still noted on the 28th day post‐CCI. Importantly, there is evidence that the above‐mentioned chemokines are also up‐regulated in other models of neuropathic pain, such as partial sciatic nerve ligation, oxaliplatin‐induced mechanical hypersensitivity and paclitaxel‐induced mechanical allodynia.46, 47, 48, 49, 50 We observed that CCL5 remained unchanged at the spinal cord level and was down‐regulated in the DRG. Some previous studies have suggested that CCL5 up‐regulation at the site of injury is a common determinant of neuropathic pain development.48 For the first time, we demonstrated elevated mRNA levels of CCL6 and CCL9 in CCI‐exposed rats. Rat CCL6 and CCL9 are orthologs of human CCL23 and CCL15, respectively.51 CCL23 is known to be involved in the pathogenesis of human brain damage52 and rheumatoid arthritis.53 Moreover, its increased level has also been observed in the cerebrospinal fluid of patients with neuropathic pain.54 In a murine model of painful diabetic neuropathy, the neutralization of CCL9 leads to the attenuation of hypersensitivity.28 These results suggest that CCR1 and the majority of its endogenous ligands are important for the development and persistence of neuropathic pain.
Hence, we decided to evaluate how the blockade of CCR1 impacts the levels of cells that are known to be strongly involved in neuropathic pain development, i.e. immune and glial cells. In rodents, CCR1 is primarily present on neutrophils,45 but it is also expressed on the surface of other types of cells.19, 20, 21, 22 Neutrophils are one of the first cells that infiltrate tissue, and they therefore take part in the inflammatory response. They are also considered to play an important role after tissue injuries.55 It is known that these granulocytes are able to infiltrate the site of injury after partial sciatic nerve lesion,56 as well as invade the DRG after CCI,57 which is in agreement with our studies. During diabetic neuropathic pain, an increased number of neutrophils might be present in the spinal cord.58 Our results provide evidence that neutrophils also infiltrate the spinal cord after CCI. After activation, they are able to release pro‐ and anti‐inflammatory factors, e.g. CCL2, CCL3, IL‐1β, IL‐1RA, IL‐4, IL‐6 and IL‐18.59 Here, we observed a strong CCI‐induced increase of MPO, a neutrophil marker that was diminished by J113863 in the DRG. Interestingly, MPO is able to activate nociceptive neurons and consequently induces pain.60 Our study indicates that a reduced level of MPO after J113863 administration was associated with the attenuation of pain‐related symptoms.
The data in the literature indicate that CCR1 is also present on CD4+ and CD8+ cells.24 The impact of T cells on neuropathic pain development has been confirmed. It was previously observed that CD4 cells may infiltrate the site of injury, the DRG and the spinal cord after peripheral nerve injury.61, 62, 63 Additionally, higher levels of CD4+ and CD8+ cells are observed in the blood after oxaliplatin and paclitaxel administration in a model of chemotherapy‐induced neuropathic pain.64 In our research, we observed an increased level of CD4+ T cells after CCI in the DRG, but not in the spinal cord. Moreover, a CCR1 antagonist significantly reduced the level of CD4+ T cells in the DRG. Some findings have shown that Rag −/− mice (the Rag gene is responsible, for example, for the formation of mature T lymphocytes) demonstrate less neuropathic pain‐like hypersensitivity after nerve injury.65 A reduction in the infiltration of T cells in combination with a decrease in the number of infiltrating neutrophils may attenuate peripherally derived pain. It seems that the modulation of the leukocyte level is an important consideration in pain pharmacotherapy. It is well known that glial cells are involved in pain development.66, 67 There are many reports showing the morphological changes of activated microglia/macrophages after nerve injury in immunofluorescence studies.68, 69 We observed, using Western blot analysis, a strong increase in the number of IBA‐1‐positive cells on the 7th day post‐CCI in the spinal cord and the DRG, which is in agreement with other data.7, 13, 15, 70, 71, 72, 73 Many of these works emphasize that the strong uncontrolled activation of microglia/macrophages is the cause of the development of neuropathic pain. In our study, a CCR1 antagonist reduced spinal microglial/macrophage activation, similarly to other substances that weakened neuropathic pain‐related symptoms, e.g. minocycline, gabapentin, zaprinast, maraviroc (CCR5 antagonist) and RS504393 (CCR2 antagonist).6, 13, 15, 74, 75 In turn, the role of GFAP‐positive cells (astroglia and satellite cells) in neuropathy is still unclear, some studies suggest that they can promote protective functions in neuropathic pain.40, 76, 77 The immunostaining against GFAP shows that astroglial cells (at the spinal cord level) and satellite cells (at DRG level) are also activated after sciatic nerve injury.7, 78, 79 The GFAP‐positive cells can control neuronal excitability and therefore their activation is beneficial for nociceptive transmission.76 In our study, J113863 did not diminish the GFAP‐positive cells activation, which can be useful. In summary, our results provide evidence that diminishing the spinal activation of IBA‐1‐positive cells, but not GFAP‐positive cells, brings beneficial effects in neuropathic pain.
We observed changes in the levels of markers of IBA‐1, CD4 and MPO, so we decided to measure the levels of selected interleukins that are known to be released by these cells after activation.76, 80 Nociceptive factors, such as interleukins, play an important role in neuropathic pain development. In our research, we observed increased levels of pronociceptive IL‐1β, IL‐6 and IL‐18 in the spinal cord and/or the DRG after chronic constriction injury, which is in agreement with other studies showing their importance in neuropathic pain.6, 81, 82, 83 J113863 markedly attenuated the levels of IL‐1β and IL‐6 in the DRG. Substances such as maraviroc14 and RS504393,13 like the presently tested CCR1 antagonist, are also able to reduce the protein levels of IL‐6 and IL‐1β in the DRG. This might be connected with a reduced level of infiltrating immune cells from the periphery, which are responsible for releasing these factors. It was previously demonstrated that i.t. administration of IL‐1β leads to the development of hypersensitivity.84 Moreover, the intraplantar injection of lipopolysaccharide or carrageenan induces pain‐related behaviors and, in parallel, elevates the levels of IL‐1β.85, 86 In contrast, an IL‐1RA is able to attenuate neuropathic pain symptoms in rats.87 Interestingly, we observed that the level of antinociceptive IL‐1RA was enhanced after chronic administration of a CCR1 antagonist compared with that in naive animals. The above‐mentioned endogenous antagonist of IL‐1R is able to blocks effects of IL‐1β.84 Similarly, Zychowska et al. 82 demonstrated that an enhanced level of IL‐1RA parallels the attenuation of CCI‐induced neuropathy symptoms. Additionally, a recombinant human IL‐1RA (Anakinra) is able to reduce pain related to rheumatoid arthritis.80 IL‐1β is mainly produced by macrophages88 but in DRG we do not observe a reduction in the protein level of IBA‐1. In our opinion decreased levels of IL‐1β might be associated with a reduced level of infiltrating immune cells, like neutrophils, which are also responsible for releasing this interleukin.59 This suggests a reduced level of MPO in the DRG after J113863 administration. What is more, we observed that the J113863 enhanced protein level of IL‐1RA, so we cannot exclude that macrophages can polarize from M1 to M2 after CCR1 antagonist, therefore the levels of IL‐1β decrease, but of IL‐1RA increase – however, this needs further study. Here, we demonstrated that a CCR1 antagonist decreased the protein level of IL‐1β and enhanced the level of IL‐1RA in the DRG, which is crucial for impaired nociceptive transmission under neuropathic pain conditions. IL‐18 also belongs to the IL‐1 superfamily.89, 90 The pronociceptive actions of IL‐18 are under the control of its naturally occurring antagonist IL‐18BP, which can be produced constitutively or due to a negative feedback mechanism.90, 91 After repeated intrathecal J113863 treatment, the spinal level of IL‐18 was decreased. Similar effects have been observed after maraviroc14 and RS50439313 administration in CCI‐exposed rats. Our results indicate that J113863 restores the balance between IL‐18 and IL‐18BP by lowering the spinal level of IL‐18 without affecting the level of IL‐18BP. The level of antinociceptive IL‐10 also remained unchanged in the spinal cord and the DRG after J113863 administration. Another important interleukin for nociceptive transmission is IL‐6. Several studies have indicated that IL‐6, which is secreted mostly by macrophages and activated microglia, is strongly increased after peripheral nerve injury. Therefore, it is considered to be one of the first factors to play a key role in neuropathic pain.84, 92, 93 In our study, we observed strongly up‐regulated IL‐6 levels in the DRG of CCI‐exposed rats and down‐regulation of this interleukin after J113863 administration. There is evidence that the neutralization of IL‐6 leads to the attenuation of hypersensitivity symptoms.94 Our results provide evidence that repeatedly administered CCR1 antagonist is able to relieve pain by reducing the activation of microglia, macrophages, neutrophils and lymphocytes in the spinal cord and/or DRG and so by inducing beneficial changes in the levels of immunological factors with pronociceptive (IL‐1β, IL‐6, and IL‐18) and antinociceptive (IL‐1RA) properties.
It was previously suggested that pronociceptive factors released from activated immune cells might attenuate opioid efficacy during neuropathic pain.6 Opioids are drugs used for the treatment of moderate to severe pain;95, 96 however, in neuropathic pain, their effectiveness is lower.97 Rojewska et al. 28 indicated that J113863 not only relieves pain‐related behavior but also improves the analgesic properties of morphine in a murine model of diabetic neuropathy, which is in agreement with our results. We demonstrated that a CCR1 antagonist enhanced the effectiveness of morphine as well as buprenorphine in CCI‐exposed rats. It has already been shown that the administration of minocycline (a microglial activation inhibitor) delays the development of morphine tolerance in naive and vehicle‐treated animals98 via the down‐regulation of IL‐6 and IL‐18 levels.6 Moreover, it has been demonstrated that the administration of IL‐1RA and IL‐18BP strengthens the analgesic efficiency of morphine and buprenorphine in CCI‐exposed rats.87, 89 We believe that the blockade of CCR1 by J113863, through a reduction in the number of IBA‐1‐positive cells and the consequent restoration of the neuroimmune balance, is one of the main mechanisms of the improved effectiveness of opioids in a CCI model. Similarly, other chemokine receptor antagonists are also able to improve opioid efficacy.13, 15 The other explanation for better opioid efficacy might be the formation of heterodimers between chemokine receptors and opioid receptors (e.g. CCR5‐MOR, CXCR2‐DOR, CXCR4‐DOR).99, 100, 101 To our knowledge, there is still no evidence that CCR1 creates dimers with opioid receptors. However, further studies are needed because it is known that CCR1 is able to heterodimerize with other chemokine receptors (e.g. CCR1–CCR5).102, 103 Moreover, there is evidence that chemokines are involved in opioid analgesic efficacy. Recent studies have demonstrated that single administration of CCL2, CCL3, CCL7 and CCL9 neutralizing antibodies can intensify the effectiveness of morphine and/or buprenorphine.28, 29, 30 These results demonstrate that chemokine systems are involved in opioid signaling and that chemokine receptor blockade may intensify the analgesic effects of opioids.
In summary, we propose CCR1 as a promising target for neuropathic pain pharmacotherapy. J113863 is able to reduce mechanical and thermal hypersensitivity after CCI and simultaneously enhance opioid efficacy. The low effectiveness of opioids in neuropathy is associated with changes in the production of pro‐ and antinociceptive factors. J113863 prevents the CCI‐induced up‐regulation of pronociceptive IL‐1β, IL‐18 and IL‐6 by reducing the activity and infiltration of IBA‐1‐positive cells, neutrophils and CD4 lymphocytes and increases the level of antinociceptive IL‐1RA. This supports the hypothesis that the pharmacological modulation of neuroimmunological interactions via CCR1 may represent a new strategy for effective polytherapy with opioids in patients suffering from neuropathic pain. Because of promising results, in our opinion, the mechanism of action consisting of blocking chemokine receptors should be considered as a target during development of new clinically used drugs. We believe that CCR1 antagonists will expand the range of commercially used medicines.
Disclosure
The authors declare that they have no conflict of interest.
Acknowledgments
KP, AP, KK, KC, KPB, WM and JM substantially contributed to the conception and design of the study, and to the analysis and interpretation of the data. This work was supported by the National Science Center, Poland, grant OPUS 11 2016/21/B/NZ4/00128 and statutory funds of the Maj Institute of Pharmacology Polish Academy of Sciences. AP and KK are PhD students funded by a scholarship from the National Center of Scientific Leading sponsored by the Ministry of Science and Higher Education, Republic of Poland. AP is supported by the Foundation for Polish Sciences, and L'Oréal Foundation and UNESCO for Women in Science. We thank Agata Ciechanowska for preparation of the graphical abstract and Katarzyna Cizio for assistance with animal work. The English was corrected by American Journal Experts (certificate no D136‐7EE8‐4085‐8CAB‐07BE).
References
- 1. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D et al Neuropathic pain. Nat Rev Dis Prim 2017; 3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schembri E. Are opioids effective in relieving neuropathic pain? SN Compr Clin Med 2019; 1:30–46. [Google Scholar]
- 3. Murnion BP. Neuropathic pain: current definition and review of drug treatment. Aust Prescr 2018; 41:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 2005; 4:834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sommer C. Peripheral neuropathies: long‐term opioid therapy in neuropathy: benefit or harm? Nat Rev Neurol 2017; 13:516–7. [DOI] [PubMed] [Google Scholar]
- 6. Rojewska E, Popiolek‐Barczyk K, Jurga AM, Makuch W, Przewlocka B, Mika J. Involvement of pro‐ and antinociceptive factors in minocycline analgesia in rat neuropathic pain model. J Neuroimmunol 2014; 277:57–66. [DOI] [PubMed] [Google Scholar]
- 7. Popiolek‐Barczyk K, Mika J. Targeting the microglial signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem 2016; 23:2908–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hung AL, Lim M, Doshi TL. Targeting cytokines for treatment of neuropathic pain. Scand J Pain 2017; 17:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brandolini L, d'Angelo M, Antonosante A, Cimini A, Allegretti M. Chemokine signaling in chemotherapy‐induced neuropathic pain. Int J Mol Sci 2019; 20:2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007; 10:1361–8. [DOI] [PubMed] [Google Scholar]
- 11. Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm 2013; 2013:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu X‐B, Jing P‐B, Zhang Z‐J, Cao D‐L, Gao M‐H, Jiang B‐C et al Chemokine receptor CCR2 contributes to neuropathic pain and the associated depression via increasing NR2B‐mediated currents in both D1 and D2 dopamine receptor‐containing medium spiny neurons in the nucleus accumbens shell. Neuropsychopharmacology 2018; 43:2320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwiatkowski K, Piotrowska A, Rojewska E, Makuch W, Mika J. The RS504393 influences the level of nociceptive factors and enhances opioid analgesic potency in neuropathic rats. J Neuroimmune Pharmacol 2017; 12:402–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piotrowska A, Kwiatkowski K, Rojewska E, Makuch W, Mika J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia – evidence from in vivo and in vitro studies. Neuropharmacology 2016; 108:207–19. [DOI] [PubMed] [Google Scholar]
- 15. Kwiatkowski K, Piotrowska A, Rojewska E, Makuch W, Jurga A, Slusarczyk J et al Beneficial properties of maraviroc on neuropathic pain development and opioid effectiveness in rats. Prog Neuropsychopharmacol Biol Psychiatry 2016; 64:68–78. [DOI] [PubMed] [Google Scholar]
- 16. Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci 2003; 4:444–55. [DOI] [PubMed] [Google Scholar]
- 17. Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res 2004; 29:1017–38. [DOI] [PubMed] [Google Scholar]
- 18. Huber AK, Giles DA, Segal BM, Irani DN. An emerging role for eotaxins in neurodegenerative disease. Clin Immunol 2018; 189:29–33. [DOI] [PubMed] [Google Scholar]
- 19. Amat M, Benjamim CF, Williams LM, Prats N, Terricabras E, Beleta J et al Pharmacological blockade of CCR1 ameliorates murine arthritis and alters cytokine networks in vivo . Br J Pharmacol 2006; 149:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 2013; 17:638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henc I, Rodzinnej EB‐FM. (2013. undefined). Chemokiny jako ważne mediatory stanu zapalnego. [WWW document]. URL https://journals.viamedica.pl/forum_medycyny_rodzinnej/article/view/36352 (accessed on 8 March 2019).
- 22. Strazza M, Mor A. Consider the chemokines: a review of the interplay between chemokines and T cell subset function. Discov Med 2017; 24:31–39. [PMC free article] [PubMed] [Google Scholar]
- 23. Shin SY, Lee DH, Lee J, Choi C, Kim J‐Y, Nam J‐S et al C‐C motif chemokine receptor 1 (CCR1) is a target of the EGF‐AKT‐mTOR‐STAT3 signaling axis in breast cancer cells. Oncotarget 2017; 8:94591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwiatkowski K, Mika J. Chemokines under neuropathic pain. Ból 2014; 15:19–35. [Google Scholar]
- 25. Gao Y‐J, Ji R‐R. Chemokines, neuronal‐glial interactions, and central processing of neuropathic pain. Pharmacol Ther 2010; 126:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kan AA, van der Hel WS, Kolk SM, Bos IWM, Verlinde SAMW, van Nieuwenhuizen O et al Prolonged increase in rat hippocampal chemokine signalling after status epilepticus. J Neuroimmunol 2012; 245:15–22. [DOI] [PubMed] [Google Scholar]
- 27. Lu P, Nakamoto Y, Nemoto‐Sasaki Y, Fujii C, Wang H, Hashii M et al Potential interaction between CCR1 and its ligand, CCL3, induced by endogenously produced interleukin‐1 in human hepatomas. Am J Pathol 2003; 162:1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rojewska E, Zychowska M, Piotrowska A, Kreiner G, Nalepa I, Mika J. Involvement of macrophage inflammatory protein‐1 family members in the development of diabetic neuropathy and their contribution to effectiveness of morphine. Front Immunol 2018; 9:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwiatkowski K, Popiolek‐Barczyk K, Piotrowska A, Rojewska E, Ciapała K, Makuch W et al Chemokines CCL2 and CCL7, but not CCL12, play a significant role in the development of pain‐related behavior and opioid‐induced analgesia. Cytokine 2019; 119:202–13. [DOI] [PubMed] [Google Scholar]
- 30. Hu J‐H, Zheng X‐Y, Yang J‐P, Wang L‐N, Ji F‐H. Involvement of spinal monocyte chemoattractant protein‐1 (MCP‐1) in cancer‐induced bone pain in rats. Neurosci Lett 2012; 517:60–3. [DOI] [PubMed] [Google Scholar]
- 31. Kiguchi N, Kobayashi Y, Maeda T, Saika F, Kishioka S. CC‐chemokine MIP‐1α in the spinal cord contributes to nerve injury‐induced neuropathic pain. Neurosci Lett 2010; 484:17–21. [DOI] [PubMed] [Google Scholar]
- 32. Matsushita K, Tozaki‐Saitoh H, Kojima C, Masuda T, Tsuda M, Inoue K et al Chemokine (C‐C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 2014; 120:1491–503. [DOI] [PubMed] [Google Scholar]
- 33. Woollard SM, Kanmogne GD. Maraviroc: a review of its use in HIV infection and beyond. Drug Des Devel Ther 2015; 9:5447–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Clercq E. Mozobil® (Plerixafor, AMD3100), 10 years after its approval by the US Food and Drug Administration. Antivir Chem Chemother 2019; 27:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16:109–10. [DOI] [PubMed] [Google Scholar]
- 36. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav 1976; 17:1031–6. [DOI] [PubMed] [Google Scholar]
- 37. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33:87–107. [DOI] [PubMed] [Google Scholar]
- 38. Rojewska E, Popiolek‐Barczyk K, Kolosowska N, Piotrowska A, Zychowska M, Makuch W et al PD98059 influences immune factors and enhances opioid analgesia in model of neuropathy. PLoS ONE 2015; 10:e0138583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makuch W, Mika J, Rojewska E, Zychowska M, Przewlocka B. Effects of selective and non‐selective inhibitors of nitric oxide synthase on morphine‐ and endomorphin‐1‐induced analgesia in acute and neuropathic pain in rats. Neuropharmacology 2013; 75:445–57. [DOI] [PubMed] [Google Scholar]
- 40. Piotrowska A, Rojewska E, Pawlik K, Kreiner G, Ciechanowska A, Makuch W et al Pharmacological blockade of CXCR3 by (±)‐NBI‐74330 reduces neuropathic pain and enhances opioid effectiveness – evidence from in vivo and in vitro studies. Biochim Biophys Acta Mol Basis Dis 2018; 1864:3418–37. [DOI] [PubMed] [Google Scholar]
- 41. Chomczynski P, Sacchi N. Single‐step method of RNA isolation by acid guanidinium thiocyanate‐phenol‐chloroform extraction. Anal Biochem 1987; 162:156–9. [DOI] [PubMed] [Google Scholar]
- 42. Mika J, Rojewska E, Makuch W, Przewlocka B. Minocycline reduces the injury‐induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience 2010; 165:1420–28. [DOI] [PubMed] [Google Scholar]
- 43. Gladue RP, Brown MF, Zwillich SH. CCR1 antagonists: what have we learned from clinical trials. Curr Top Med Chem 2010; 10:1268–77. [DOI] [PubMed] [Google Scholar]
- 44. Pevida M, Lastra A, Meana Á, Hidalgo A, Baamonde A, Menéndez L. The chemokine CCL5 induces CCR1‐mediated hyperalgesia in mice inoculated with NCTC 2472 tumoral cells. Neuroscience 2014; 259:113–25. [DOI] [PubMed] [Google Scholar]
- 45. Lewis ND, Muthukumarana A, Fogal SE, Corradini L, Stefanopoulos DE, Adusumalli P et al CCR1 plays a critical role in modulating pain through hematopoietic and non‐hematopoietic cells. PLoS ONE 2014; 9:e105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saika F, Kiguchi N, Kobayashi Y, Fukazawa Y, Kishioka S. CC‐chemokine ligand 4/macrophage inflammatory protein‐1β participates in the induction of neuropathic pain after peripheral nerve injury. Eur J Pain 2012; 16:1271–80. [DOI] [PubMed] [Google Scholar]
- 47. Illias AM, Gist AC, Zhang H, Kosturakis AK, Dougherty PM. Chemokine CCL2 and its receptor CCR2 in the dorsal root ganglion contribute to oxaliplatin‐induced mechanical hypersensitivity. Pain 2018; 159:1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liou J‐T, Mao C‐C, Ching‐Wah Sum D, Liu F‐C, Lai Y‐S, Li J‐C et al Peritoneal administration of Met‐RANTES attenuates inflammatory and nociceptive responses in a murine neuropathic pain model. J Pain 2013; 14:24–35. [DOI] [PubMed] [Google Scholar]
- 49. Ochi‐Ishi R, Nagata K, Inoue T, Tozaki‐Saitoh H, Tsuda M, Inoue K. Involvement of the chemokine CCL3 and the purinoceptor P2×7 in the spinal cord in paclitaxel‐induced mechanical allodynia. Mol Pain 2014; 10:10–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ke BC, Huang XX, Li Y, Li LY, Xu QX, Gao Y et al Neuronal‐derived Ccl7 drives neuropathic pain by promoting astrocyte proliferation. NeuroReport 2016; 27:849–57. [DOI] [PubMed] [Google Scholar]
- 51. Alliance of Genome Resources , http://www.alliancegenome.org [accessed on 7 October 2019]
- 52. Simats A, García‐Berrocoso T, Penalba A, Giralt D, Llovera G, Jiang Y et al CCL23: a new CC chemokine involved in human brain damage. J Intern Med 2018; 283:461–75. [DOI] [PubMed] [Google Scholar]
- 53. Rioja I, Hughes FJ, Sharp CH, Warnock LC, Montgomery DS, Akil M et al Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor α, tumor necrosis factor receptor superfamily member 9, and macrophage colony‐stimulating factor. Arthritis Rheum 2008; 58:2257–67. [DOI] [PubMed] [Google Scholar]
- 54. Bäckryd E, Lind A‐L, Thulin M, Larsson A, Gerdle B, Gordh T. High levels of cerebrospinal fluid chemokines point to the presence of neuroinflammation in peripheral neuropathic pain: a cross‐sectional study of 2 cohorts of patients compared with healthy controls. Pain 2017; 158:2487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu F‐C, Chuang Y‐H, Tsai Y‐F, Yu H‐P. Role of neutrophil extracellular traps following injury. Shock 2014; 41:491–8. [DOI] [PubMed] [Google Scholar]
- 56. Perkins NM, Tracey DJ. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience 2000; 101:745–57. [DOI] [PubMed] [Google Scholar]
- 57. Morin N, Owolabi S, Harty M, Papa E, Tracy T Jr, Shaw S et al Neutrophils invade lumbar dorsal root ganglia after chronic constriction injury of the sciatic nerve. J Neuroimmunol 2007; 184:164–171. [DOI] [PubMed] [Google Scholar]
- 58. Newton VL, Guck JD, Cotter MA, Cameron NE, Gardiner NJ. Neutrophils infiltrate the spinal cord parenchyma of rats with experimental diabetic neuropathy. J Diabetes Res 2017; 2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tecchio C, Micheletti A, Cassatella MA. Neutrophil‐derived cytokines: facts beyond expression. Front Immunol 2014; 5:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rosas EC, Correa LB, das Graças Henriques M. Neutrophils in rheumatoid arthritis: a target for discovering new therapies based on natural products In: Maitham AK, ed. Role of Neutrophils in Disease Pathogenesis. InTech, 2017; 5:89–118. [Google Scholar]
- 61. Zhao H, Alam A, Chen Q, Eusman MA, Pal A, Eguchi S et al The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth 2017; 118:504–16. [DOI] [PubMed] [Google Scholar]
- 62. Austin PJ, Kim CF, Perera CJ, Moalem‐Taylor G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 2012; 153:1916–31. [DOI] [PubMed] [Google Scholar]
- 63. Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004; 129:767–77. [DOI] [PubMed] [Google Scholar]
- 64. Makker PGS, Duffy SS, Lees JG, Perera CJ, Tonkin RS, Butovsky O et al Characterisation of immune and neuroinflammatory changes associated with chemotherapy‐induced peripheral neuropathy. PLoS ONE 2017; 12:e0170814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bravo‐Caparrós I, Nieto FR. Roles for CD8+ T cells and IL‐10 in the resolution of paclitaxel‐induced neuropathic pain. J Neurosci 2017; 37:2803–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 2010; 10:167–84. [DOI] [PubMed] [Google Scholar]
- 67. Romero‐Sandoval A, Chai N, Nutile‐McMenemy N, DeLeo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res 2008; 1219:116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig 2016; 7:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018; 100:1292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Popiolek‐Barczyk K, Kolosowska N, Piotrowska A, Makuch W, Rojewska E, Jurga AM et al Parthenolide relieves pain and promotes M2 microglia/macrophage polarization in rat model of neuropathy. Neural Plast 2015; 2015:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wen Y‐R, Tan P‐H, Cheng J‐K, Liu Y‐C, Ji R‐R. Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance. J Formos Med Assoc 2011; 110:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep 2008; 60:297–307. [PubMed] [Google Scholar]
- 73. Leinders M, Knaepen L, De Kock M, Sommer C, Hermans E, Deumens R. Up‐regulation of spinal microglial Iba‐1 expression persists after resolution of neuropathic pain hypersensitivity. Neurosci Lett 2013; 554:146–50. [DOI] [PubMed] [Google Scholar]
- 74. Yang J‐L, Xu B, Li S‐S, Zhang W‐S, Xu H, Deng X‐M et al Gabapentin reduces CX3CL1 signaling and blocks spinal microglial activation in monoarthritic rats. Mol Brain 2012; 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rojewska E, Piotrowska A, Jurga A, Makuch W, Mika J. Zaprinast diminished pain and enhanced opioid analgesia in a rat neuropathic pain model. Eur J Pharmacol 2018; 839:21–32. [DOI] [PubMed] [Google Scholar]
- 76. Mika J, Zychowska M, Popiolek‐Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol 2013; 716:106–19. [DOI] [PubMed] [Google Scholar]
- 77. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009; 10:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mika J, Osikowicz M, Rojewska E, Korostynski M, Wawrzczak‐Bargiela A, Przewlocki R et al Differential activation of spinal microglial and astroglial cells in a mouse model of peripheral neuropathic pain. Eur J Pharmacol 2009; 623:65–72. [DOI] [PubMed] [Google Scholar]
- 79. Deng Z, Li C, Du E, Liu C, Xia B, Chen H et al Catestatin enhances neuropathic pain mediated by P2X 4 receptor of dorsal root ganglia in a rat model of chronic constriction injury. Cell Physiol Biochem 2018; 51:812–26. [DOI] [PubMed] [Google Scholar]
- 80. Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010; 16:1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang B, Zhang G, Yang M, Liu N, Li Y‐X, Ma H et al Neuroprotective effect of anethole against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Neurochem Res 2018; 43:2404–22. [DOI] [PubMed] [Google Scholar]
- 82. Zychowska M, Rojewska E, Makuch W, Luvisetto S, Pavone F, Marinelli S et al Participation of pro‐ and anti‐nociceptive interleukins in botulinum toxin A‐induced analgesia in a rat model of neuropathic pain. Eur J Pharmacol 2016; 791:377–88. [DOI] [PubMed] [Google Scholar]
- 83. Gui W‐S, Wei X, Mai C‐L, Murugan M, Wu L‐J, Xin W‐J et al Interleukin‐1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain 2016; 12:174480691664678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mika J, Korostynski M, Kaminska D, Wawrzczak‐Bargiela A, Osikowicz M, Makuch W et al Interleukin‐1α has antiallodynic and antihyperalgesic activities in a rat neuropathic pain model. Pain 2008; 138:587–97. [DOI] [PubMed] [Google Scholar]
- 85. Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000; 4:247–57. [DOI] [PubMed] [Google Scholar]
- 86. Ren K, Torres R. Role of interleukin‐1β during pain and inflammation. Brain Res Rev 2009; 60:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pilat D, Rojewska E, Jurga AM, Piotrowska A, Makuch W, Przewlocka B et al IL‐1 receptor antagonist improves morphine and buprenorphine efficacy in a rat neuropathic pain model. Eur J Pharmacol 2015; 764:240–8. [DOI] [PubMed] [Google Scholar]
- 88. Rivera Vargas T, Martin F, Apetoh L. Role of interleukin‐1‐family cytokines on effector CD4 T cell differentiation. World J Immunol 2017; 7:24. [Google Scholar]
- 89. Pilat D, Piotrowska A, Rojewska E, Jurga A, Ślusarczyk J, Makuch W et al Blockade of IL‐18 signaling diminished neuropathic pain and enhanced the efficacy of morphine and buprenorphine. Mol Cell Neurosci 2016; 71:114–24. [DOI] [PubMed] [Google Scholar]
- 90. Samarani S, Allam O, Sagala P, Aldabah Z, Jenabian M‐A, Mehraj V et al Imbalanced production of IL‐18 and its antagonist in human diseases, and its implications for HIV‐1 infection. Cytokine 2016; 82:38–51. [DOI] [PubMed] [Google Scholar]
- 91. Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin‐18 and IL‐18 binding protein. Front Immunol 2013; 4:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. DeLeo JA, Colburn RW, Nichols M, Malhotra A. Interleukin‐6‐mediated hyperalgesia/allodynia and increased spinal IL‐6 expression in a rat mononeuropathy. Model J Interf Cytokine Res 1996; 16:695–700. [DOI] [PubMed] [Google Scholar]
- 93. Lee K‐M, Jeon S‐M, Cho H‐J. Interleukin‐6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK. Eur J Pain 2010; 14:682.e1–12. [DOI] [PubMed] [Google Scholar]
- 94. Zhou Y‐Q, Liu Z, Liu Z‐H, Chen S‐P, Li M, Shahveranov A et al Interleukin‐6: an emerging regulator of pathological pain. J Neuroinflammation 2016; 13:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wordliczek J, Dobrogowski J, Wszechświat RZ. (2013. undefined) Zastosowanie leków opioidowych w leczeniu bólu. [WWW document]. URL http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.agro-6d20ceea-3cb6-4fcb-b693-6173d43f4915/c/Jerzy_Wordliczek.pdf(accessed on 6 March 2019).
- 96. Szczudlik A, Dobrogowski J, Wordliczek J, Stępień A, Krajnik M, Leppert W et al Rozpoznanie i leczenie bólu neuropatycznego: przegląd piśmiennictwa i zalecenia Polskiego Towarzystwa Badania Bólu i Towarzystwa Neurologicznego‐część pierwsza* Diagnosis and management of neuropathic pain: review of literature and recommendations of the Polish Association for the Study of Pain and the Polish Neurological Society – part one. Tom 2014; 15:8–18. [Google Scholar]
- 97. Kwiatkowski K, Mika J. The importance of chemokines in neuropathic pain development and opioid analgesic potency. Pharmacol Rep 2018; 70:821–30. [DOI] [PubMed] [Google Scholar]
- 98. Mika J, Wawrzczak‐Bargiela A, Osikowicz M, Makuch W, Przewlocka B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun 2009; 23:75–84. [DOI] [PubMed] [Google Scholar]
- 99. Parenty G, Appelbe S, Milligan G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of the CXCR2‐DOP receptor heterodimer. Biochem J 2008; 412:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Parsadaniantz SM, Rivat C, Rostène W, Goazigo AR‐L. Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nat Rev Neurosci 2015; 16:69–78. [DOI] [PubMed] [Google Scholar]
- 101. Arnatt CK, Falls BA, Yuan Y, Raborg TJ, Masvekar RR, El‐Hage N et al Exploration of bivalent ligands targeting putative mu opioid receptor and chemokine receptor CCR5 dimerization. Bioorg Med Chem 2016; 24:5969–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kramp BK, Megens RTA, Sarabi A, Winkler S, Projahn D, Weber C et al Exchange of extracellular domains of CCR1 and CCR5 reveals confined functions in CCL5‐mediated cell recruitment. Thromb Haemost 2013; 110:795–806. [DOI] [PubMed] [Google Scholar]
- 103. Di Prisco S, Summa M, Chellakudam V, Rossi PIA, Pittaluga A. RANTES‐mediated control of excitatory amino acid release in mouse spinal cord. J Neurochem 2012; 121:428–37. [DOI] [PubMed] [Google Scholar]


