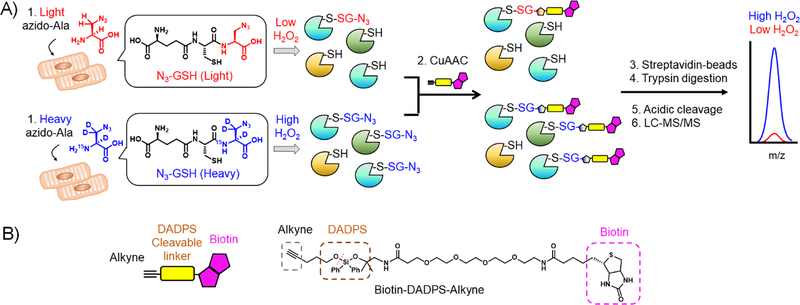
Figure 1. Isotopic-labeling strategy to identify and quantify glutathionylated cysteines.
(A) A scheme for quantification of glutathionylated peptides. A mutant of glutathione synthetase (GS M4) in HL-1 cells uses light or heavy azido-Ala to synthesize isotopically-labelled clickable glutathione. Upon addition of stimulus, two cohorts of lysates containing glutathionylated proteins with a light or heavy label were combined and subjected to click reaction with biotin-DADPS-alkyne, pull-down by streptavidin-beads, and tryptic digestion. Glutathionylated peptides were eluted by acidic cleavage of a DADPS linker and analyzed by LC-MS/MS, which provides a MS1-peak area ratio (RH/L) of heavy- to light-labelled peptides. (B) The structure of biotin-DADPS-alkyne with its cleavage site (dot line).