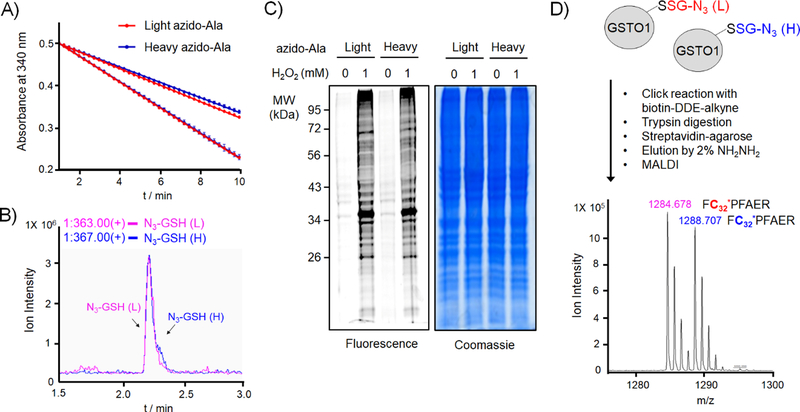
Figure 2. Evaluation of light and heavy derivatives of azido-Ala to identify glutathionylated peptides with isotopic mass difference.
(A) Light and heavy azido-Ala derivatives are used equally by GS M4 in vitro. GS M4 enzyme activity was measured with γ-Glu-Cys and ATP in the presence of two different concentrations (0.2 and 1 mM) of light azido-Ala (red) or heavy azido-Ala (blue), showing identical enzymatic rates. (B) Light and heavy azido-Ala derivatives produce the equal amount of respective azido-glutathione in cells. HEK293 expressing GS M4 (HEK293/GS M4) were incubated with light or heavy azido-Ala. Lysates were analyzed by LC-MS to detect the mass of light or heavy-labelled azido-glutathione. (C) Light and heavy azido-Ala derivatives detect the identical pattern of glutathionylation. HEK293/GS M4 cells incubated with light or heavy azido-Ala were treated with H2O2 (1 mM, 15 min). Lysates were then subjected to click reaction with Cy5-alkyne for fluorescence detection or Coomassie stains. (D) Light and heavy azido-Ala derivatives identify glutathionylated peptides with isotopic mass difference. Purified GSTO1 was glutathionylated by addition of diamide (0.2 mM, 30 min) in the presence of equal amount of light- and heavy-labelled azido-glutathione derivatives (both 1 mM). Glutathionylated GSTO1 was processed for click reaction with biotin-DDE-alkyne, tryptic digestion, pull-down, elution, and MALDI-MS analysis.[12, 20] Data are representative of 3 independent experiments.