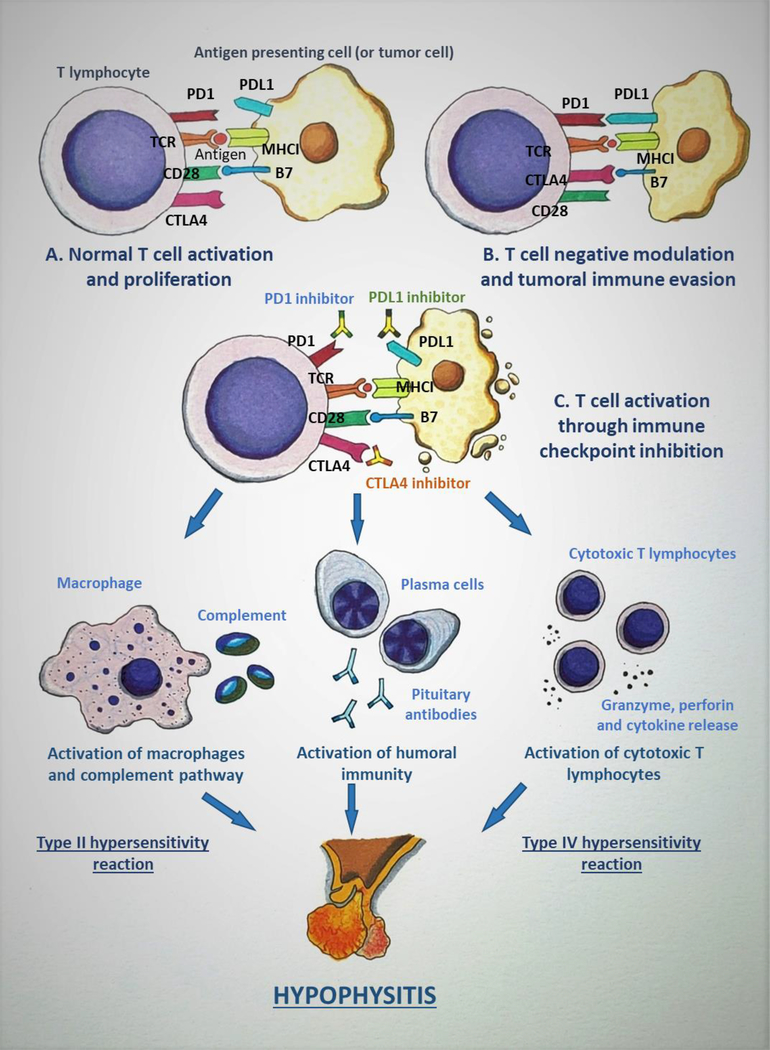
Figure 1:
Mechanism of immune checkpoint inhibitor-induced hypophysitis (ICIH). A: The tumor antigen is presented to T lymphocyte (TLC) by antigen presenting cell (APC), which in this scenario can be a tumor cell, through the interaction between the T-cell receptor (TCR) and the major histocompatibility complex I (MHCI). Another simultaneous interaction occurs between CD28 on the TLC and B7 on the APC which leads to the completion of TLC activation. B: Cytotoxic T-lymphocyte antigen 4 (CTLA4) is a negative immunomodulator that is induced after the initial activation of TLC. CTLA4 binds to B7 with a higher affinity when compared to CD28, and causes inactivation of TLC. Programmed death 1 (PD1) is up-regulated in activated TLCs and, in response to the inflammatory state, various tissues express PD1 ligand 1 (PDL1) and the PD1/PDL1 interaction down-regulates TLC activation. These mechanisms act as immune checkpoints and are utilized by normal cells in the body to prevent immune-mediated destruction. But the tumor cells take advantage of these immunomodulatory pathways to evade the immune system. C: Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target the immune checkpoints (CTLA4, PD1/PDL1) and inhibit their action, leading to persistent TLC activation and proliferation. This up-regulation in TLC activity caused by ICIs leads to destruction of the tumor and, as an adverse effect, also causes ICIH and other immune-related adverse events. The binding of ICI to pituitary cells elicits various immune reactions: a type II hypersensitivity reaction leading to activation of macrophages and complement pathways, a type IV hypersensitivity reaction causing cytotoxic T lymphocyte activation resulting in the release of granzyme, perforin, and cytokines, and activation of humoral immunity pathway leading to the production of anti-pituitary antibodies, all of which cause the disruption of the pituitary gland. (Courtesy of Sriram Gubbi, NIDDK, NIH)