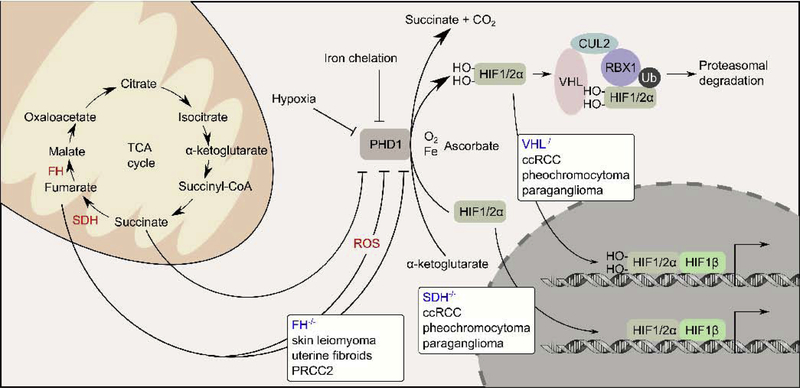
Figure 2. VHL−/−, SDH−/−, and FH−/− are implicated in pseudohypoxia response.
Under normoxia conditions, HIF1A and HIF2A are hydroxylated by prolyl hydroxylase (PHD1), a reaction that requires molecular oxygen, iron, and α-ketoglutarate as co-factors. VHL is a substrate adapter protein that mediates the ubiquitylation of the hydroxylated forms of HIF1A and HIF2A by a cullin 2 (CUL2) containing E3-ubiquitin ligase complex. The ubiquitylated HIF1A and HIF2A are targeted for proteasomal degradation. VHL−/− causes the hydroxylated forms of HIF1A and HIF2A to accumulate, allowing them to activate target genes under normaxia condition, a phenomenon known as pseudohypoxia. VHL−/− causes ccRCC, pheochromocytoma, and paraganglioma. Like VHL−/−, SDH−/− also causes ccRCC, pheochromocytoma, and paraganglioma. In SDH−/− tumors, succinate accumulates and acts as a competitive inhibitor of PHD1, causing the accumulation and activation of HIF1A and HIF2A, and pseudohypoxia. Unlike VHL−/− and SDH−/−, FH−/− causes a different spectrum of tumors (skin leiomyoma, uterine fibroids, and PRCC2). Several studies demonstrated that fumarate accumulates following FH inactivation and can drive pseudohypoxia by either competitively inhibiting PHD1 or by generating reactive oxygen species, which in turn inhibits PHD1.