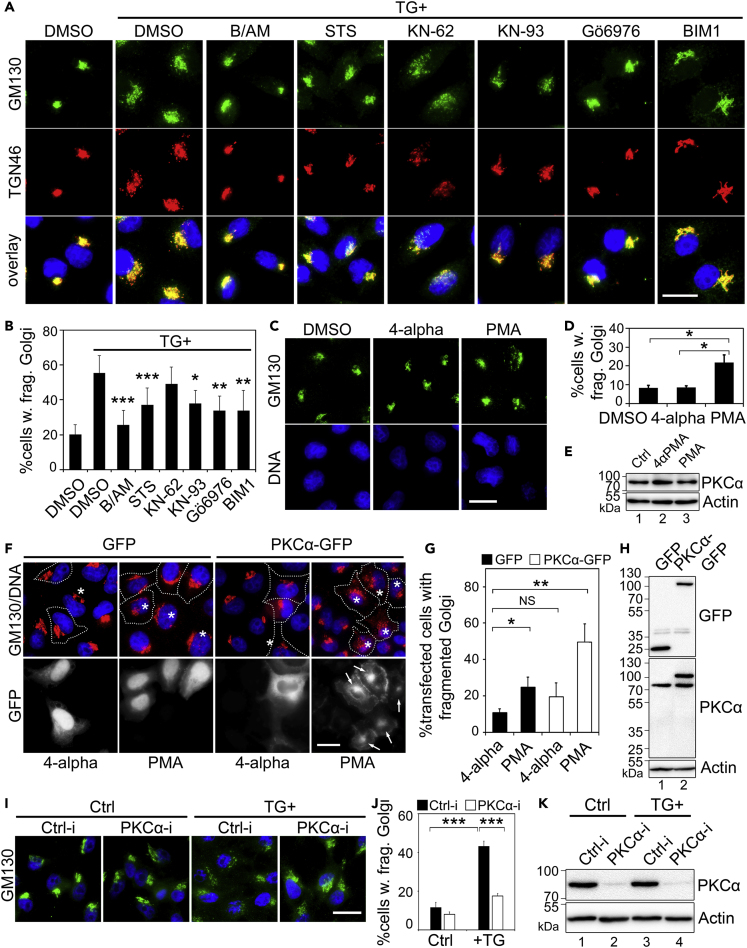
Figure 4.
TG induces Golgi Fragmentation through PKCα
(A) Inhibition of PKC reduces TG-induced Golgi fragmentation. HeLa cells were pre-treated with DMSO, BAPTA-AM (B/AM, 60 μM for 10 min), staurosporine (STS, general kinase inhibitor, 2 μM for 10 min), KN-62 or KN-93 (CAMKII inhibitors, 10 μM and 5 μM, respectively, 10 min), or BIM1 or Gӧ6976 (PKC inhibitors, 2 μM and 4 μM, respectively, 10 min), and then with 250 nM TG for 20 min followed by immunostaining of GM130 and TGN46. Scale bar, 20 μm.
(B) Quantitation of cells in (A) with fragmented Golgi based on the GM130 pattern in the cell.
(C) HeLa cells were treated with 100 nM PMA for 1 h; 4-alpha-PMA of the same concentration was used as a control. Scale bar, 20 µm.
(D) Quantitation of cells with fragmented Golgi in (C).
(E) Cells treated as in (C) were analyzed by Western blot to show that PMA treatment does not change the PKCα expression level.
(F) Activation of ectopically expressed PKCα triggers its Golgi localization (indicated by “→”) and Golgi fragmentation (∗). Cells transfected with GFP or PKCα-GFP were treated with 100 nM PMA or 4-alpha for 1 h. Note the fragmented Golgi in these cells upon PMA treatment. Scale bar, 20 μm.
(G) Quantitation of cells in (F) with fragmented Golgi.
(H) Western blot of cells from (F) showing that ectopic PKC expression does not alter the endogenous PKCα expression level.
(I) HeLa cells were transfected with control (Ctrl-i) or PKC-specific siRNA for 48 h and then treated with 250 nM TG for 20 min. Cells were fixed and stained for GM130 (green) to show the Golgi structure. Scale bar, 20 μm.
(J) Quantitation of Golgi fragmentation of cells in (I).
(K) Cells in (I) were blotted for endogenous PKCα to evaluate the siRNA knockdown efficiency.
All quantitation results are shown as Mean ± SEM from three independent experiments. Statistical analyses were performed using two-tailed Student's t-tests (∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; NS, non-significant).