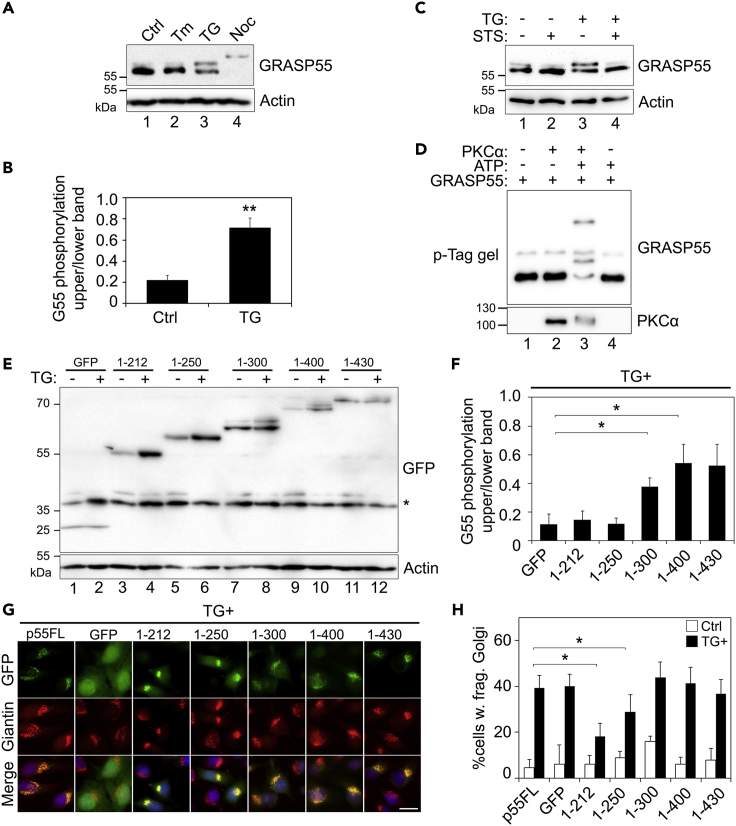
Figure 5.
TG Induces Golgi Fragmentation through GRASP55 Phosphorylation
(A) GRASP55 is phosphorylated upon TG treatment. HeLa cells treated with Tm, TG, or nocodazole (Noc) were analyzed by phos-tag gels and Western blot. Note the mobility shift of GRASP55 upon TG treatment compared with control (Ctrl). Nocodazole-arrested mitotic cells were used as a positive control for GRASP55 phosphorylation.
(B) TG-induced GRASP55 mobility shift is abolished by kinase inhibition. Cells were pre-treated with 2 μM staurosporine (STS) for 10 min and then with 250 nM TG for 1 h followed by analysis with phos-tag gels and Western blot.
(C) Quantitation of GRASP55 phosphorylation in TG-treated cells. The intensity of the phosphorylated (upper) band was quantified by densitometry analysis and plotted relative to the intensities of the full length (lower) band. Shown are the results of relative phosphorylation of GRASP55 from three independent experiments.
(D) PKCα phosphorylates GRASP55 in vitro. Purified PKCα and GRASP55 were incubated in a kinase buffer with or without ATP as indicated and analyzed by phos-tag gels and Western blot for GRASP55 and PKCα.
(E) Mapping the phosphorylation site on GRASP55 by expressing GRASP55 truncation mutants. Indicated GFP-tagged GRASP55 constructs were expressed in HeLa cells. After TG treatment, GRASP55 was analyzed by mobility shift as in (A). Note the mobility shift in lanes 8, 10, and 12.
(F) Quantitation of GRASP55 phosphorylation in TG-treated cells from (E).
(G) TG-induced Golgi fragmentation is rescued by the expression of non-phosphorylatable GRASP55 proteins. Cells expressing the indicated GRASP55 constructs were stained for giantin. Scale bar, 20 µm.
(H) Quantitation of cells in (G) with fragmented Golgi. Results are shown as Mean ± SEM.
Statistical analyses were performed using two-tailed Student's t-tests (∗p ≤ 0.05; ∗∗p ≤ 0.01).