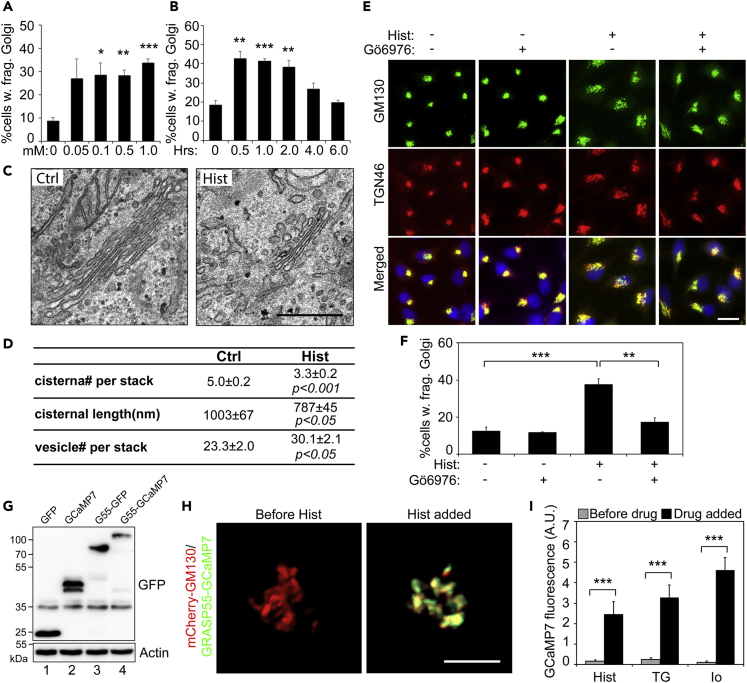
Figure 6.
Histamine Induces Golgi Fragmentation through PKC Activation
(A) HeLa cells were treated with indicated concentrations of histamine for 1 h, stained for GM130, and quantified for the percentage of cells with fragmented Golgi. Shown are the quantitation results.
(B) HeLa cells were treated with 100 μM histamine for the indicated times and analyzed as in (A). Note that histamine induced Golgi fragmentation within 2 h.
(C) Electron micrographs of Golgi profiles in HeLa cells treated with DMSO control (Ctrl, left panel) or 100 μM histamine for 1 h (right panel). Note the reduced size of the Golgi in histamine-treated cells. Scale bar, 0.5 μm.
(D) Quantitation of the morphological features of the Golgi stacks in (C). For statistics, histamine treated cells were compared with control cells.
(E) Cells were pre-treated with (+) or without (−) the PKC inhibitor Gö6976 at 37°C for 10 min followed by the addition of 100 μM histamine for 60 min. Scale bar, 20 μm.
(F) Quantitation of cells with fragmented Golgi in (E).
(G) Expression of the GRASP55-GCaMP7 Golgi Ca2+ sensor. HeLa cells transfected with indicated proteins were analyzed by Western blot for GFP.
(H) Histamine treatment increases the GRASP55-GCaMP7 signal. HeLa cells were co-transfected with mCherry-GM130 (red) and GRASP55-GCAMP7 (green). Shown are still frames before (left panel) or after (right) histamine was added. Scale bar, 5 µm.
(I) TG and ionomycin treatments increase the GRASP55-GCaMP7 signal. HeLa cells expressing GRASP55-GCaMP7 were treated with 100 μM histamine (Hist), 250 nM TG, or 1 μM ionomycin (Io) for 1 h. Shown are the quantitation of the fluorescence intensity before and after the drug was added.
All quantitation results are shown as Mean ± SEM. Statistical analyses were performed using two-tailed Student's t-tests (∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001).