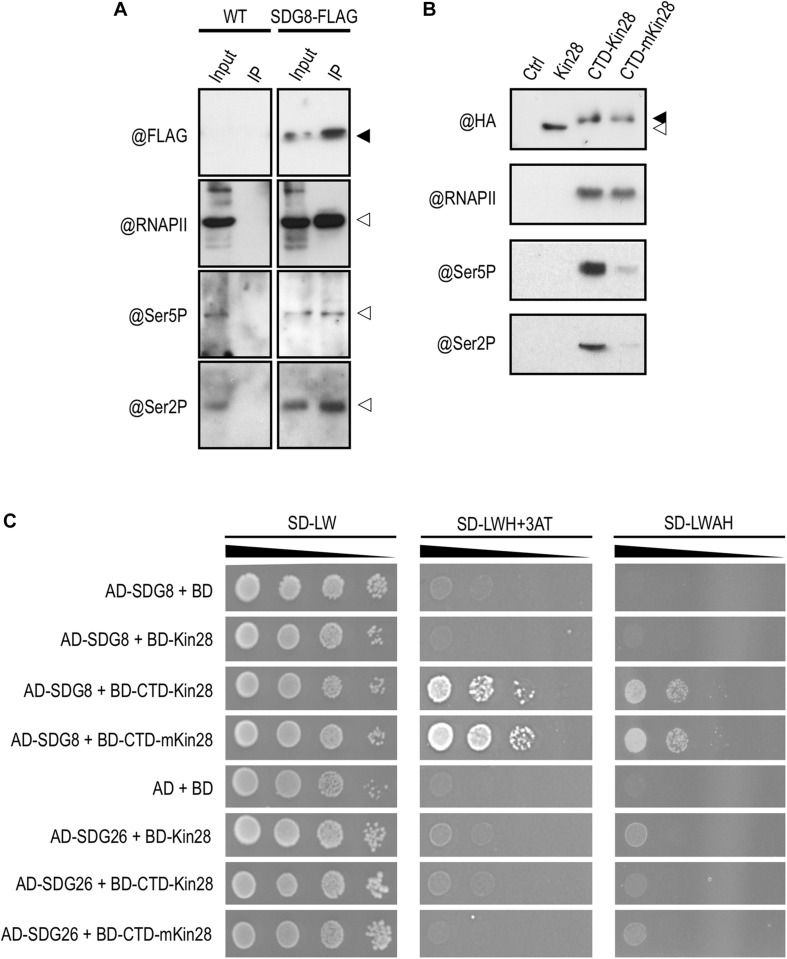
FIGURE 6.
Protein interaction between SDG8 and different phosphorylated forms of RNAPII. (A) Proteins were extracted from wild-type (WT) and plants expressing a functional FLAG-tagged SDG8 protein in a sdg8 mutant background (SDG8-FLAG). An anti-FLAG antibody was used to detect the tagged SDG8 protein (black arrowhead) in the input and after immunoprecipitation using anti-FLAG beads (IP). RNAPII (@RNAPII), the Ser5P form of the CTD of RNAPII (@Ser5P) or the Ser2P form of the CTD of RNAPII (@Ser2P) in input and after IP are indicated by white arrowheads. The experiments were repeated at least twice with similar results. (B) The two-hybrid system used to identify protein-protein interactions requiring post-translational modifications was tested for the phosphorylation of the CTD. An anti-HA antibody was used to detect fusion protein in untransformed yeast (Ctrl) and in yeast transformed with the protein kinase Kin28 alone (white arrowhead) or with the CTD fused to either Kin28 (CTD-Kin28) or the inactive Kin28 (CTD-mKin28). Phosphorylation of the CTD detected by antibodies against the Ser5P form of the CTD (@Ser5P) or the Ser2P form of the CTD (@Ser2P) occurs mainly with the tethered wild-type Kin28 but not with the mutated one. The experiments were repeated twice with similar results. (C) Yeast two-hybrid analysis of the interaction between SDG8 and the phosphorylated or non-phosphorylated form of the RNAPII CTD. Full-length SDG8 and SDG26 were separately fused to the Gal4 Activation domain (AD), while Kin28, CTD-Kin28 and CTD-mKin28 were fused to the Gal4 DNA Binding domain (BD). Yeast cells co-transformed with the different recombinant vectors were spotted though a series of 10-fold dilutions onto control (SD-LW) and selection media of increasing stringency (SD-LWH + 3-AT and SD-LWAH). The experiments were repeated twice with similar results.