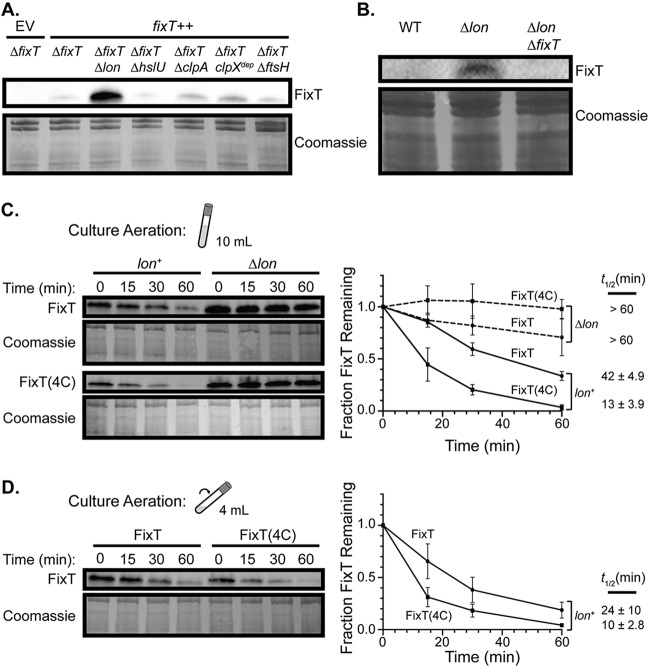
FIG 5.
The Lon protease degrades FixT in vivo. (A) Western blot of lysates from strains lacking ATP-dependent protease systems probed with anti-FixT antibody. Strains lack chromosomal fixT and carry either an empty vector (EV) or a fixT overexpression plasmid (fixT++). Coomassie stain of the membrane is shown as a loading control. (B) Western blot using an anti-FixT antibody to assess steady-state levels of endogenously expressed fixT in wild-type (WT), Δlon mutant, and ΔfixT Δlon mutant cell lysates under saturating microaerobic conditions. The Coomassie stain of the membrane is shown as a loading control. (C) In vivo degradation experiments using exponential-phase cells grown under limited aeration (determined by culture volume and tilt depicted in cartoon). Left, representative anti-FixT Western blots of ΔfixT (lon+) mutant and ΔfixT Δlon (Δlon) mutant cells overexpressing fixT or fixT(4C). Cells were sampled at the indicated intervals after arresting translation with oxytetracycline. Coomassie stains of membranes are shown as a loading control. Right, quantification of FixT levels from replicates of the experiment shown to the left (average ± SD, n = 3). The half-life (t1/2; average ± SD, n = 3) of FixT in each strain is displayed to the right of the graph. The difference between FixT and FixT(4C) half-lives in the lon+ background is significant (P < 0.005, Student’s t test). (D) In vivo degradation experiments as in panel C using exponential-phase cells grown under enhanced aeration (smaller volume and greater tilt angle, as shown in cartoon). Left, representative anti-FixT Western blot of ΔfixT (lon+) mutant cells overexpressing either fixT or fixT(4C). Right, quantification of FixT levels from replicates of the experiment shown to the left. Points and half-lives are shown as the averages ± SD, n = 3.