Abstract
Introduction
Radiation therapy is one of the most common tools for treating cancer. The aim is to deliver adequate doses of radiation to kill cancer cells and the most challenging part during this procedure is to protect normal cells from radiation. One strategy is to use a radioprotector to spare normal tissues from ionizing radiation effects. Researchers have pursued cerium oxide nanoparticles as a therapeutic agent, due to its diverse characteristics, which include antioxidant properties, making it a potential radioprotector.
Materials and methods
One hundred rats were divided into five groups of A) control group, intraperitoneal (IP) saline injection was done twice a week; B) bi-weekly IP injection of 14.5 nM (0.00001 mg/kg) CNP for two weeks; C) a single whole thorax radiation dose of 18 Gy; D) a single whole thorax radiation dose of 18 Gy + bi-weekly injection of 14.5 nM CNP for two weeks after radiation; E) bi-weekly IP injection of 14.5 nM CNP for two weeks prior to radiation + a single whole thorax radiation dose of 18 Gy. Thirty days after irradiation, 7 rats from each group were anesthetized and their lungs extracted for histopathological examination.
Results
Statistical analyses revealed that CNP significantly decreased the incidence of tissue collapse and neutrophile aggregation in rats receiving CNP before radiation in comparison with the radiation group.
Conclusion
The results suggested the possibility of using CNP as a future radioprotector due to its ability to protect normal cells against radiation-induced damage.
Keywords: Radiation, Cerium oxide nanoparticle, Lung
1. Introduction
Although decades have passed since the advent of nuclear age and its promises to improve living conditions, there are still challenges in relation to appropriate use of nuclear or radiologic technology. Regardless of whether these exposures are for medical procedures, the need for effective actions to confront radiation health hazards is warranted.
Time, distance and shielding are basic guides for radiation protection, included in every radiation health plan. However, there are certain conditions in which employing these principles are next to impossible. In certain conditions, regardless of whether these principles are applied or not, there is still a need for more protection. The word “radioprotector” is labeled for every equipment or material (medicine, nutritional supplements or engineered devices) that is used before or during exposure to prevent the development or escalation of radiation injuries.1,2
Nanotechnology has many applications, and in recent years has been the focus of biomedical studies. One of the greatest services that nanotechnology can provide is to facilitate and promote healthcare system and upgrade biomedical research.3 In at least one dimension, nanoparticles (NPs) are 100 nm in size, whereas the sizes of cells are 10,000−20,000 nm; hence, they can easily enter a cell and react with organelles and proteins.
In addition, nanoparticles (NPs) characteristics in nanoscale, makes them a good candidate for biological and therapeutic uses.4
Cerium oxide nanoparticle (CNP) has been used in some industries, such as electronic, automobile, polishing, and energy. Recently, it has been shown that it can be used in biology and the healthcare system due to its features.5 Several studies have shown various roles of CNP as anti-inflammatory, anti-cancer, free radical scavenging, anti-obesity and wound repairing.6, 7, 8, 9, 10, 11, 12, 13
CNP has shown to have antioxidant or pro-oxidant properties. Several studies have showed CNP as a radioprotector and others introduced it as a radiosensitizer.14, 15, 16, 17 A reason for this phenomenon is the pH of the environment. In neutral pH, CNP shows its antioxidant properties, but in acidic pH, it might become pro-oxidant. Moreover, a few studies have showed that ROS inhibition activity is also related to Ph. Since normal cell environment is neutral and cancer cell environment is acidic; hence, we think CNP can be useful in radiotherapy. This can help to protect normal cells and sensitization of cancer cells in order to achieve our aim in radiotherapy.11,18, 19, 20 The aim of radiotherapy is to deliver enough doses of radiation to kill cancer cells. The most challenging part of this procedure is to protect normal cells from radiation.18
A unique feature of CNP is its regenerative antioxidant property. Cerium oxide can have two forms: CeO2 and Ce2O3. Coexistence of Ce3+ and Ce4+ (without structural changes) enables these particles to interact with free radicals due to oxygen defects or reactive sites on their surface.11,21 They can store and release oxygen, and after one cycle, they can participate in another cycle and by this feature, it can be more effective with less amount of particles.21
In this study, we evaluated the radioprotection effect of CNP in rats’ lungs, exposed to 18 Gy whole-thorax X-ray. CNP was given to rats before and after radiation. Thirty days after radiation we surveyed histopathological effects. The aim was to discuss whether CNP has lowered these effects or not.
2. Materials and methods
2.1. Animals
Healthy adult male Sprague-Dawley rats were purchased from the center of comparative and experimental medicine, Shiraz University of Medical Sciences, Shiraz, Iran. The rats weighing 220−250 g were housed in the university animal house based on the guidelines of “The Guide for the Care and Use of Laboratory Animals” prepared by SUMS. These principles include characteristics of animal's natural life in captivity, using spacious cage, preparing appropriate ventilation and light, handling with care, and providing water and food. All animals were kept under controlled conditions (25 ± 2 °C), humidity (55 ± 5%) and light (12 h of light and dark cycle). Each five animals were housed in polypropylene cages, containing sterile husk bedding throughout the experiments. This study was approved by the local ethics committee of SUMS. Rats were kept in animal house for 140 days after irradiation for survival monitoring. After 140 days all survived animals were sacrificed using ketamine and xylazine injection.
2.2. Nanoparticle
Cerium oxide nanopowder was purchased from US research nanomaterials, inc. (Houston, TX77084, USA) containing 10 g of CeO2, 99.97%, the size of CNP was between 10−30 nm.
According to manufacturers’ instruction, CeO2 nanopowder was dissolved in distilled water (pH 7) and diluted to 14.5 nM (according to Colon et al.22).
2.3. Experimental design
One hundred rats were divided into five groups: A) control group, intraperitoneal (IP) saline injection was done twice a week for two weeks. This group underwent 4 injections of saline to experience the stress of injection similar to experiment groups; B) bi-weekly IP injection of 14.5 nM (0.00001 mg/kg) CNP for two weeks. Comparison of this group to control group determines the effect of CNP on the lung and survival rate of this group shows the toxicity of CNP; C) a single whole thorax radiation dose of 18 Gy (without any other intervention); D) a single whole thorax radiation dose of 18 Gy + bi-weekly injection of 14.5 nM CNP for two weeks after radiation, their first injection was 30 min after radiation. This group was designed to determine the radioprotection effect of CNP; E) bi-weekly IP injection of 14.5 nM CNP for two weeks prior to radiation + a single whole thorax radiation dose of 18 Gy. Their last injection was 30 min before radiation. This group was designed to determine the radiomitigating effect of CNP.
Due to limitations in working with high numbers of rats, repetition of experiments was impractical.
2.4. Irradiation
Before radiation exposure, rats were anesthetized using ketamine 10% at a dose of 80 mg/kg and xylazine 2% at a dose of 5 mg/kg with an IP injection. Rats were placed in a supine position and their head and body, except their thorax, was shielded. A 1 cm thick tray for buildup was placed 1 cm from the skin. SSD was 117 cm and the dose rate was 200 MU/min. 2520 MU in total was given.
The X-ray system (Electa accelerator, 6 MeV) in the radiotherapy department of the Namazee hospital, Shiraz, Iran, was employed to provide a single radiation dose of 18 Gy.
2.5. Pathology
Thirty days after irradiation, 7 rats out of each group were selected randomly. Rats were anesthetized using ketamine and xylazine, then their lungs were extracted for histopathologic examinations. Lungs were kept in 10% formalin and embedded in Paraffin wax. Sections were cut at 5 μm thickness, processed and stained with Hematoxylin and Eosin (H&E). The slides were evaluated under a light microscope (ZEISS, standard 20, Germany) by a pathologist blinded to the study, at the Ali-Asghar hospital, Shiraz, Iran.
In effort to evaluate the radiation-induced injuries in lung tissues, we assessed 7 parameters including: presence of neutrophils, lymphocytes, macrophages, and incidence of erythrocytes (red blood cell [RBC]), hyaline arteriosclerosis, edema and collapse, and scored these variables from 1 to 4 (according to Haddadi et al.23). On this scale, 1 is no change, 2 mild change, 3 moderate, and 4 shows severe damage.
2.6. Statistical analysis
Data were analyzed using IBM SPSS Statistical software version 25. Kaplan–Meier method was performed to evaluate the survival rate. Histopathologic data were analyzed using the Kruskal–Wallis test and one-way ANOVA was used for mean values, and its results are presented as mean ± standard deviation. P < 0.05 was considered to be statistically significant.
3. Results
For each rat 7 pathologic parameters were observed and we gave a grade from 1 to 4 according to the severity of the damage.
3.1. Survival
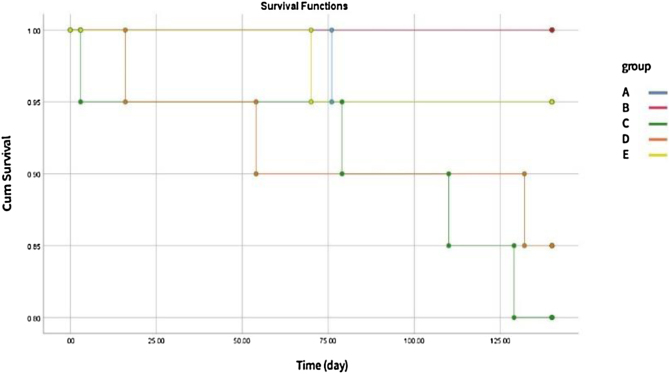
In an attempt to administer and evaluate the radiation protection effect of CNP on live animals, the rats’ survival rate was measured. Rats were exposed to thorax18 Gy X-ray in the presence or absence of bi-weekly IP injection of CNP for two weeks before or after irradiation, and they were monitored for 140 days. We excluded 7 rats which were sacrificed after 30 days from survival analyses. Fig. 1 illustrates the result of survival analysis. Results showed that CNP is well tolerated by rats. Group B rats that only received CNP were all alive until the last day.
Fig. 1.
Rats survival rate monitored for 140 days after irradiation. A) control group (1 death), B) CNP group (no death), C) radiation group (4 deaths), D) radiation + CNP after radiation (3 deaths), E) CNP before radiation + radiation (1 death).
Results showed that CNP is well tolerated by rats. Group B that only received CNP, were all alive until the last day.
3.2. Histopathology
Table 1 shows the mean value ± standard deviation of each variable in each group.
Table 1.
Effect of CNP 30 days post-irradiation on histopathological factors in the lung tissue of rats. Mean ± standard deviation is shown in five groups. A: Control, B: bi-weekly injection of CNP for two weeks, C: 18 Gy radiation group, D: 18 Gy irradiation + bi-weekly IP injection of CNP for two weeks after irradiation, E: bi-weekly IP injection of CNP for two weeks before irradiation +18 Gy irradiation.
| Groups | A | B | C | D | E |
|---|---|---|---|---|---|
| Injury | |||||
| Edema | 1.42 ± 0.53 | 1.28 ± 0.48 | 2.57 ± 0.53 | 2.00 ± 081 | 1.71 ± 0.75 |
| Lymphocyte | 1.85 ± 0.37 | 1.85 ± 0.37 | 3.28 ± 0.48 | 2.71 ± 0.48 | 2.28 ± 0.75 |
| Neutrophile | 1.00 ± 0.00 | 1.00 ± 0.00 | 2.42 ± 0.78 | 1.42 ± 0.53 | 1.14 ± 0.37 |
| Macrophage | 1.57 ± 0.53 | 1.57 ± 0.53 | 2.71 ± 0.48 | 2.14 ± 0.69 | 2.28 ± 0.48 |
| RBC | 1.57 ± 0.53 | 1.85 ± 0.37 | 2.28 ± 1.11 | 2.14 ± 0.69 | 2.00 ± 0.81 |
| Collapse | 1.28 ± 0.48 | 1.14 ± 0.37 | 2.85 ± 0.69 | 1.71 ± 0.75 | 1.42 ± 0.53 |
| Hyaline arteriosclerosis | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.14 ± 0.37 | 1.14 ± 0.37 | 1.00 ± 0.00 |
Amongst all the groups, the radiation group obtained the highest score in all parameters. Group B receiving only CNP, was identical to the control group regarding the amount of lymphocyte, neutrophile, macrophage, and hyaline arteriosclerosis. CNP reduced edema and collapse in comparison with the control group, but increased RBC. Generally, administration of CNP before or after radiation, reduced every parameter when compared to the radiation group, but the reduction rate was greater in group E, that received CNP before radiation. However, the differences between survival rates of groups were not statically significant.
Kruskal–Wallis test showed no significant difference in RBC and hyaline arteriosclerosis between the groups. Pairwise comparison for other variables showed that incidence of edema was significantly different between group B and the radiation group (P = 0.017). As the results indicate, the number of macrophages was significantly higher in the radiation group when compared with the control group (P = 0.016) and CNP group (P = 0.016). in addition, aggregation of lymphocytes was significantly higher in the radiation group in comparison with the control and the CNP group (P = 0.02, P = 0.02). The differences between other groups lacked significance.
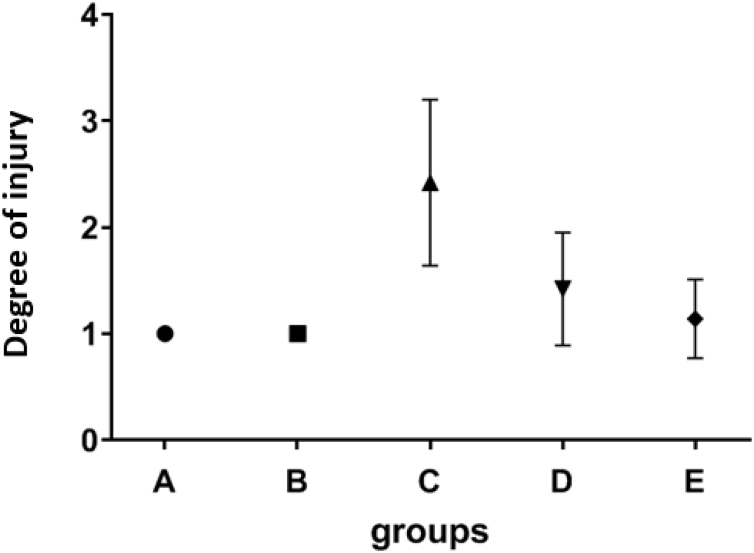
Statistical results showed that the number of neutrophils in radiation group was significantly higher than the control and the CNP group (P = 0.004, P = 0.004), group E that received CNP before radiation, also had significantly less number of neutrophils than the radiation group (P = 0.014), but no significant difference was observed between the radiation group and rats that received CNP after radiation (Fig. 2).
Fig. 2.
The effect of CNP on neutrophile aggregation. Mean values and standard deviations are shown. A: Control group, B: bi-weekly injection of CNP for two weeks, C: 18 Gy radiation group, D: 18 Gy irradiation + bi-weekly IP injection of CNP for two weeks after irradiation, E: bi-weekly IP injection of CNP for two weeks before irradiation +18 Gy irradiation.
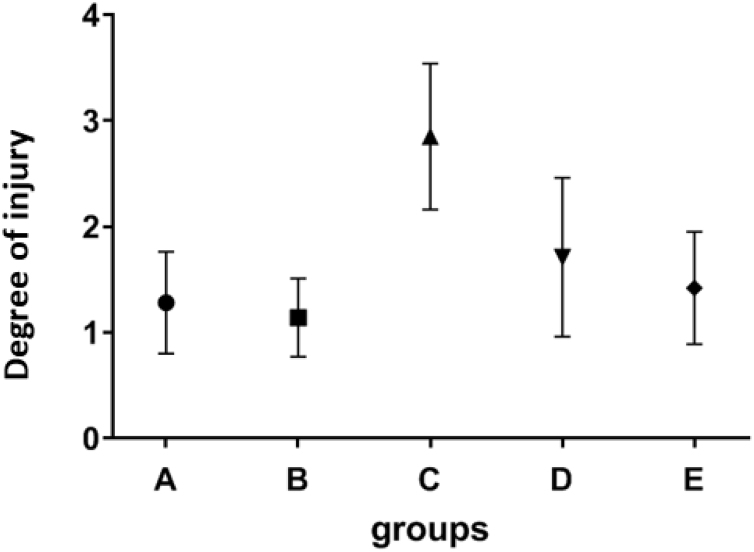
Collapse incidence was significantly different between the control and radiation group (P = 0.009), between CNP group and radiation group (P = 0.002). Injecting CNP before radiation, significantly reduced the collapse in comparison with the radiation group (P = 0.038). No other statistically significant differences were observed between the groups (Fig. 3).
Fig. 3.
The effect of CNP on incidence of radiation-induced collapse. Mean values and standard deviations are shown. A: Control group, B: bi-weekly injection of CNP for two weeks, C: 18 Gy radiation group, D: 18 Gy irradiation + bi-weekly IP injection of CNP for two weeks after irradiation, E: bi-weekly IP injection of CNP for two weeks before irradiation +18 Gy irradiation.
4. Discussion
Ionizing radiation can create free radicals and ROS, causing cellular oxidative damage. These changes can lead to molecular alteration or mitotic death. In lung tissue, radiation toxicity increases edema, permeability and aggregation of inflammatory cells and macrophages.24 When cells are exposed to radiation, they try to decrease the effects of free radicals by strengthening repair and protective mechanisms. Despite these efforts, they cannot completely combat cell damages and might lead toward cell death. In order to overcome radiation effects, researchers have studied free radical scavengers extensively, and some radioprotectors, such as Amifostine, melatonin and vitamin E, have been introduced, but each of them has particular limitations in clinical usage.25
In this study, we evaluated the radioprotection effect of CNP in rats, exposed to thorax single dose of 18 Gy X-ray. To achieve this, we surveyed radiation-induced histopathological changes in lung tissue. Results indicated that radiation increased edema, collapse and the number of lymphocytes, neutrophils, and macrophages. The group that received CNP before radiation, had a significantly lower number of neutrophils and less collapse. Hence, we can conclude that CNP had a protecting effect against radiation-induced lung tissue injuries.
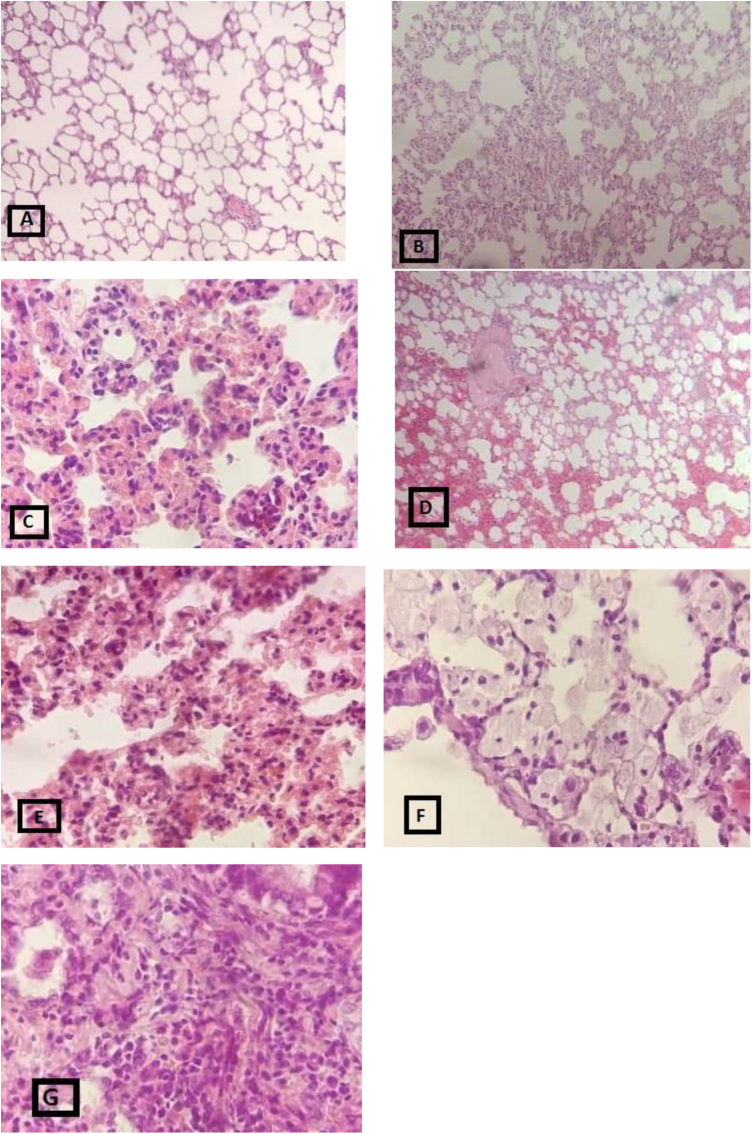
The protection effect of CNP against radiation-induced pathologic damages was reported before. For instance, Colon et al., 2009, reported that CNP protected lung tissue from radiation-induced pneumonitis. They used 15 nM CNP, 15 and 18 Gy whole-thorax radiation and they compared CNP with their Amifostine group and found out that CNP decreased fibrosis and collagen deposition in comparison with the radiation and Amifostine group.22 In another study by Madero et al., 2012, H&E staining in irradiated head and neck region cells also showed the preservation of cells in comparison with the radiation alone group which had a vast lymphocyte and macrophage invasion and morphological damage.26 Our experimental design including the radiation dose, molarity, and size of CNP, was somewhat similar to the above-mentioned studies. Their results were in line with ours, confirming that CNP might have protective effect by decreasing the radiation-induced pathologic damages (Fig. 4).
Fig. 4.
Histopathologic evaluation of radiation-induced lung damages, extracted 30 days after irradiation, using H&E staining showing: A: no change, B: collapse, C: edema, D: hyaline arteriosclerosis E: lymphocytes, F: macrophage, G: neutrophile.
Xu et al., 2016, used H&E and Masson’s trichrome staining to evaluate the degree of fibrosis and pneumonitis in mice treated with CNP-18 and CNP-ME (different in synthetic method) as a radiomitigator and scored the damage on scale of 0-8. They reported that the radiation + CNP-18 group showed significantly less radiation-induced pneumonitis and fibrosis compared to the radiation group. Their result indicated that in case of injecting CNP after radiation, it can have a mitigating effect.27 Accordingly, we also compared the radioprotective effect of CNP when injected before or after radiation. Even though we clearly observed that mean values in group D were less than the radiation group and closer to our control group, this data lacked significance. However, there was a significant protection against collapse and number of neutrophils, when CNP was injected before radiation. Also in other parameters, mean values for group E were lower than group D. According to the results, we are of the opinion that injecting CNP before and after radiation might be effective, but if it is injected before radiation, it might lead to better protection.
In contrast to our findings, few studies have reported the toxicity5,28,29 or even radiosensitizing effect of CNP,30 but we should consider that many parameters can influence the results. The most important parameter is the cell line because CNP can have a different effect in acidic conditions. The studies which showed a radiosensitizing effect of CNP were performed on cancer cells with acidic environment.30,31 Other parameters included dose and size of CNP, synthetic method, crystal structure and storage duration that affected the results.
Evaluating the radioprotection effect of CNP and by comparing this effect when it was injected before and after radiation under the same condition, and also scoring the histopathological damages, were the positive points of our study. Nonetheless, there were some limitations. The size of CNP used in this study was 10−30 nm (the smallest size that we could provide), but it would have been better to use a size under 10 nm according to the studies that obtained better results. Also, more samples might help to achieve a more reliable result and future studies to elucidate the protective effect of CNP on long-term radiation-induced damages are warranted.
5. Conclusion
The results of the present study showed that CNP can reduce some pathologic damages of lung radiation and suggest the possibility of using CNP as a future radioprotector due to its ability to protect normal cells against radiation-induced pathologic damages. Considering these results and the results of similar studies, we can conclude that CNP might be a promising radioprotector in clinical applications. Additional studies on delivery methods and experiments to fully elucidate the mechanism of action of CNP are required.
Conflict of interest
None declared.
Financial disclosure
This paper has been extracted from the results of the MSc thesis of Fatemeh Kadivar (No.14498) and grant (No.15560) that was funded by Research Council at Shiraz University of Medical Sciences, Shiraz, Iran.
Contributor Information
Fatemeh Kadivar, Email: kadivar.fateme@gmail.com.
Gholamhassan Haddadi, Email: pira_radio4@sums.ac.ir.
Mohammad Amin Mosleh-Shirazi, Email: mosleh_amin@hotmail.com.
Fatemeh Khajeh, Email: mojgankhaje@gmail.com.
Alireza Tavasoli, Email: Tavaspath790@yahoo.com.
References
- 1.Seed T.M. Radiation protectants: current status and future prospects. Health Phys. 2005;89(5):531–545. doi: 10.1097/01.hp.0000175153.19745.25. [DOI] [PubMed] [Google Scholar]
- 2.Kuntić V.S., Stanković M.B., Vujić Z.B., Brborić J.S., S.M.J.C Uskoković‐Marković. Radioprotectors—the evergreen topic. Biodiversity. 2013;10(10):1791–1803. doi: 10.1002/cbdv.201300054. [DOI] [PubMed] [Google Scholar]
- 3.Sahoo S., Parveen S., Panda J. The present and future of nanotechnology in human health care. Nanomed Nanotechnol Biol Med. 2007;3(1):20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura H., Watano S. Direct permeation of nanoparticles across cell membrane: a review. KONA Powder Part J. 2018;35:49–65. [Google Scholar]
- 5.Rice K.M., Nalabotu S.K., Manne N.D. Exposure to cerium oxide nanoparticles is associated with activation of mitogen-activated protein kinases signaling and apoptosis in rat lungs. J Prev Med Public Health. 2015;48(3):132. doi: 10.3961/jpmph.15.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirst S.M., Karakoti A.S., Tyler R.D., Sriranganathan N., Seal S., Reilly C.M. Anti‐inflammatory properties of cerium oxide nanoparticles. Small. 2009;5(24):2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 7.Nelson B., Johnson M., Walker M., Riley K., Sims C. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants. 2016;5(2):15. doi: 10.3390/antiox5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagliari F., Mandoli C., Forte G. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6(5):3767–3775. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- 9.Chigurupati S., Mughal M.R., Okun E. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34(9):2194–2201. doi: 10.1016/j.biomaterials.2012.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca A., Moscato S., Ronca F. Pilot in vivo investigation of cerium oxide nanoparticles as a novel anti-obesity pharmaceutical formulation. Nanomed Nanotechnol Biol Med. 2015;11(7):1725–1734. doi: 10.1016/j.nano.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Pešić M., Podolski-Renić A., Stojković S. Anti-cancer effects of cerium oxide nanoparticles and its intracellular redox activity. Chem Biol Interact. 2015;232:85–93. doi: 10.1016/j.cbi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Rim K.T., Koo K.H., Park J.S. Toxicological evaluations of rare earths and their health impacts to workers: a literature review. Saf Health Work. 2013;4(1):12–26. doi: 10.5491/SHAW.2013.4.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodea-Palomares I., Gonzalo S., Santiago-Morales J. An insight into the mechanisms of nanoceria toxicity in aquatic photosynthetic organisms. Aquat Toxicol. 2012;122:133–143. doi: 10.1016/j.aquatox.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Azizi S., Ghasemi A., Asgarian-Omran H. Cerium oxide nanoparticles sensitize non-small lung cancer cell to ionizing radiation. Marmara Pharm J. 2018;22(2) [Google Scholar]
- 15.Baker C.H. Harnessing cerium oxide nanoparticles to protect normal tissue from radiation damage. Transl Cancer Res. 2013;2(4):343–358. [Google Scholar]
- 16.Montazeri A., Zal Z., Ghasemi A., Yazdannejat H., Asgarian-Omran H., Hosseinimehr S.J. Radiosensitizing effect of cerium oxide nanoparticles on human leukemia cells. Pharm Nanotechnol. 2018;6(2):111–115. doi: 10.2174/2211738506666180306161253. [DOI] [PubMed] [Google Scholar]
- 17.Al-Mashhadani A.H., Yas R.M. Study of in vitro and in vivo free radical scavenging activity for radioprotection of cerium oxide nanoparticles. Iraqi J Phys. 2017;15(35):40–47. [Google Scholar]
- 18.Vazirov R., Sokovnin S., Ilves V., Myshkina A., Bazhukova I., editors. AIP conference proceedings. AIP Publishing; 2018. Application of cerium oxide nanoparticles as modificators in radiation therapy. [Google Scholar]
- 19.Wason M.S., Colon J., Das S. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomed Nanotechnol Biol Med. 2013;9(4):558–569. doi: 10.1016/j.nano.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehman M.U., Jawaid P., Kondo T. Dual effects of nanoparticles on radiation therapy: as radiosensitizers and radioprotectors. Radiat Environ Med. 2016;5:40–45. [Google Scholar]
- 21.Lee T.-L., Raitano J.M., Rennert O.M., Chan S.-W., Chan W.-Y. Accessing the genomic effects of naked nanoceria in murine neuronal cells. Nanomed Nanotechnol Biol Med. 2012;8(5):599–608. doi: 10.1016/j.nano.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colon J., Herrera L., Smith J. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5(2):225–231. doi: 10.1016/j.nano.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Haddadi G.H., Rezaeyan A., Mosleh-Shirazi M.A. Hesperidin as radioprotector against radiation-induced lung damage in rat: a histopathological study. J Med Phys. 2017;42(1):25. doi: 10.4103/jmp.JMP_119_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks L.B., Yu X., Vujaskovic Z., Small W. Jr, Folz R., Anscher M.S., editors. Seminars in radiation oncology. Elsevier; 2003. Radiation-induced lung injury. [DOI] [PubMed] [Google Scholar]
- 25.Gudkov S., Popova N., Bruskov V.J.B. Radioprotectors: history, trends and prospects. Biofizika. 2015;60(4):801–811. [PubMed] [Google Scholar]
- 26.Madero-Visbal R.A., Alvarado B.E., Colon J.F. Harnessing nanoparticles to improve toxicity after head and neck radiation. Nanomedicine. 2012;8(7):1223–1231. doi: 10.1016/j.nano.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Xu P., Maidment B., 3rd, Antonic V. Cerium oxide nanoparticles: a potential medical countermeasure to mitigate radiation-induced lung injury in CBA/J mice. Radiat Res. 2016;185(5):516–526. doi: 10.1667/RR14261.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin W., Huang Y.-w, Zhou X.-D., Ma Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006;25(6):451–457. doi: 10.1080/10915810600959543. [DOI] [PubMed] [Google Scholar]
- 29.Aalapati S., Ganapathy S., Manapuram S., Anumolu G., Prakya B.M.J.N. Toxicity and bio-accumulation of inhaled cerium oxide nanoparticles in CD1 mice. Nanotoxicology. 2014;8(7):786–798. doi: 10.3109/17435390.2013.829877. [DOI] [PubMed] [Google Scholar]
- 30.Wason M.S., Colon J., Das S. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine. 2013;9(4):558–569. doi: 10.1016/j.nano.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y., Chen K., Ma J.-l., Gao F.J.O. Cerium oxide nanoparticles in cancer. Onco Targets Ther. 2014;7:835. doi: 10.2147/OTT.S62057. [DOI] [PMC free article] [PubMed] [Google Scholar]