Abstract
Objectives
Tricuspid valve disease is increasingly encountered, but surgery is rarely performed in isolation, in part because of a reported higher operative risk than other single-valve operations. Although guidelines recommend valve repair, there is sparse literature for the optimal surgical approach in isolated tricuspid valve disease. We performed a meta-analysis examining outcomes of isolated tricuspid valve repair versus replacement.
Methods
We searched Pubmed, Embase, Scopus and Cochrane from January 1980 to June 2019 for studies reporting outcomes of both isolated tricuspid valve repair and replacement, excluding congenital tricuspid aetiologies. Data were extracted and pooled using random-effects models and Review Manager 5.3 software.
Results
There were 811 article abstracts screened, from which 52 full-text articles reviewed and 16 studies included, totalling 6808 repairs and 8261 replacements. Mean age ranged from 36 to 68 years and females made up 24%–92% of these studies. Pooled operative mortality rates and odds ratios (95% confidence intervals) for isolated tricuspid repair and replacement surgery were 8.4% vs 9.9%, 0.80 (0.64 to 1.00). Tricuspid repair was also associated with lower in-hospital acute renal failure 12.4% vs 15.6%, 0.82 (0.72 to 0.93) and pacemaker implantation 9.4% vs 21.0%, 0.37 (0.24 to 0.58), but higher stroke rate 1.5% vs 0.9%, 1.63 (1.10 to 2.41). There were no differences in rates of prolonged ventilation, mediastinitis, return to operating room or late mortality.
Conclusion
Isolated tricuspid valve repair was associated with significantly reduced in-hospital mortality, renal failure and pacemaker implantation compared with replacement and is therefore recommended where feasible for isolated tricuspid valve disease, although its higher stroke rate warrants further research.
Keywords: cardiac surgery, tricuspid valve disease, surgery-valve
Key questions.
What is already known about this subject?
Isolated tricuspid valve surgery is uncommonly performed making up to 20% of tricuspid valve surgery, because of poor outcomes compared with other single valve surgery. Surgical repair and replacement are two strategies to address isolated severe tricuspid valve disease with mixed results from the literature.
What does this study add?
This is the first meta-analysis comparing isolated tricuspid valve repair and replacement, pooling 16 cohort studies and 15 069 patients. We found that isolated tricuspid valve repair was associated with reduced operative mortality (8.4% vs 9.9%) as well as lower rates of renal failure and pacemaker implantation, but a higher rate of stroke.
How might this impact on clinical practice?
The findings support recommendations of performing surgical repair where feasible in isolated tricuspid valve disease. Isolated tricuspid surgery remains a high risk procedure regardless of whether repair or replacement is undertaken. Given this clinical scenario being increasingly encountered, percutaneous tricuspid valve interventions, if effective, may play an important role in the future to fill this unmet need.
Introduction
The ‘forgotten’ tricuspid valve has lost its label over the last decade due to increased recognition that severe tricuspid regurgitation is associated with worse prognosis.1–3 The gold-standard treatment remains open heart surgery, indicated in those with severe tricuspid valve disease who present with either symptoms of right sided heart failure or progressive right ventricular dilation or systolic dysfunction.1 4 The threshold for performing isolated tricuspid valve surgery is higher in part because of the markedly higher operative mortality risk compared with other single valve surgery, approximately 9% vs 2%–3%.5 6 This presents a challenging clinical decision, and isolated tricuspid valve surgery only makes up 20% of all tricuspid valve surgeries.7 Although guidelines suggest that tricuspid repair is preferred when feasible over replacement similar to for mitral valve surgery, this is based on a limited number of observation studies only.1 To address this, we performed a meta-analysis of the outcomes of patients undergoing isolated tricuspid valve repair versus replacement.
Methods
Search criteria
Ethical approval was not required for the conduct of this meta-analysis with no individual-level patient data collected. The PRISMA guidelines for meta-analysis protocol were used for this study. Medline, Embase, Scopus and Cochrane electronic databases and reference lists of relevant searched articles during 1 January 1980 to 30 June 2019 were searched for adult human original clinical studies comparing tricuspid valve repair with replacement. The search terms used were (tricuspid) AND (repair OR annulopasty) AND (replacement OR bioprosthetic OR mechanical). Isolated tricuspid valve surgery was defined as either surgical repair or replacement of the tricuspid valve without concurrent valvular heart surgery within the same procedure (such as mitral or aortic valve operations). For inclusion, studies needed to report separate outcomes for isolated tricuspid valve repair and replacement arms and have at least 10 patients in each group. Studies describing exclusively congenital tricuspid valve disease warranting surgery were excluded. Reviews, editorials and guidelines were excluded.
Data collection
For all studies meeting the inclusion criteria, data were extracted for analysis separately for valve repair and replacement. Study characteristics recorded include author and year of publication, surgical cohort date and location, number of patients undergoing surgical repair and replacement, aetiology of tricuspid valve disease requiring surgery, demographics (age and sex) and follow-up duration. The primary outcomes of interest were operative mortality defined as in-hospital and/or within 30 days. Other outcomes studied include in-hospital morbidities and longer-term mortality after the operative period during follow-up.
Statistical analysis
Review Manager V.5.3 (Cochrane Collaboration, Oxford, England) was used for performing pooled analysis. Estimated effect measures were performed for binary outcomes using the Mantel-Haenszel statistical method and random effect models. The OR with 95% CI calculated in this study are based on the odds of an event for tricuspid valve repair compared with replacement, for example, OR less than 1.0 indicates lower event rate for surgical repair. Outcomes are only pooled if they are reported by over three studies. Heterogeneity of the outcomes reported across studies were assessing using the Cochrane Q χ² statistic and I2 statistic, the former with p values and the latter considered significant heterogeneity if >50%. Funnel plots were used to evaluate for publication bias. The significance level was set at 5% and all tests were two-tailed. The meta-analysis was performed using the PRISMA guidelines.
Results
A total of 811 entries were obtained from the literature search, and figure 1 summarises the studies disposition. After abstract screening, 54 full-text studies were evaluated before arriving at the final 16 studies that met the inclusion criteria of this meta-analysis.6–21Table 1 lists the main characteristics of the included studies, totalling 6808 isolated tricuspid valve repairs and 8261 isolated tricuspid valve replacement patients. The range of average age was 36–68 years, females comprised 24%–92% and follow-up ranged from in-hospital/30 days to 19.1 years.
Figure 1.
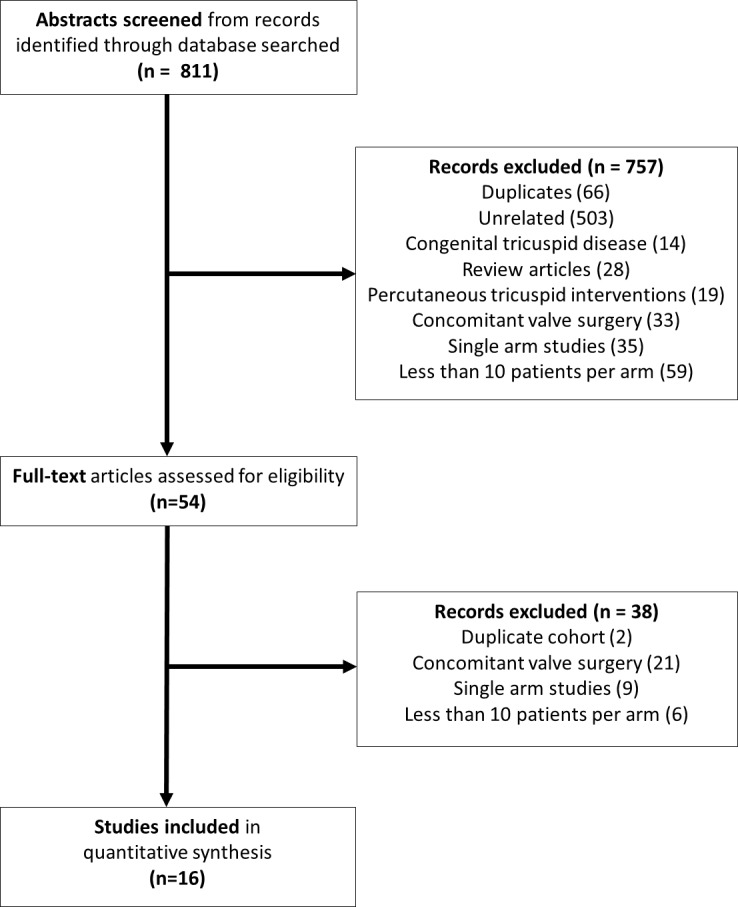
Literature search disposition.
Table 1.
Characteristics of included studies of isolated tricuspid valve surgery
| Author(s) | Year | Country | Cohort date | Repair | Replacement | Aetiology | Age (years) | Female (%) | Follow-up |
| Alkhouli et al8 | 2018 | USA | Jan 2003–Dec 2014 | 569 | 765 | TR secondary | 55 | 56 | In-hospital |
| Axtell et al9 | 2019 | USA | Nov 2001–Mar 2016 | 143 | 28 | All TR | 61 | 54 | 2.6 years |
| Chen et al10 | 2019 | China | Jan 2005–Aug 2018 | 25 | 93 | TR past LHS | 55 | 79 | 2.7 years |
| Di Mauro et al11 | 2019 | Italy | 1983–2018 | 77 | 80 | TV IE | 47 | 24 | 19.1 years |
| Ejiofor et al12 | 2017 | USA | Jan 2002–Mar 2014 | 18 | 39 | All TV | 55 | 61 | 3.5 years |
| Farag et al13 | 2017 | Germany | 1995–2011 | 41 | 68 | All TR | 53 | 50 | N/A (long-term) |
| Gaca et al14 | 2013 | USA | 2002–2009 | 354 | 490 | TV IE | 41 | 49 | 30 days |
| Hamandi et al15 | 2019 | USA | 2007–2017 | 68 | 27 | All TV | 56 | 66 | N/A (long-term) |
| Kim et al16 | 2013 | South Korea | Sep 1996–July 2010 | 37 | 14 | All TR | 55 | 51 | 4.0 years |
| Kundi et al17 | 2019 | USA | 2003–2014 | 2494 | 2670 | All TV | 68 | 58 | 1.0 year |
| Moutakiallah et al18 | 2018 | Morocco | 2018 | 15 | 11 | TR RHD | 48 | 92 | 4.6 years |
| Oh et al19 | 2013 | New Zealand | 1965–2011 | 38 | 34 | All TV | 48 | 71 | 13.7 years |
| Protos et al20 | 2018 | USA | 2012–2016 | 12 | 26 | TV IE | 36 | 47 | 1.0 year |
| Raikhelkar et al21 | 2012 | USA | Nov 1998–Nov 2010 | 27 | 29 | All TV | 56 | 57 | 2.3 years |
| Vassileva et al7 | 2012 | USA | 1999–2008 | 2465 | 3271 | All TV | N/A | N/A | In-hospital |
| Zack et al6 | 2017 | USA | 2004–2013 | 425 | 616 | All TV | 60 | 58 | In-hospital |
IE, infective endocarditis; LHS, left heart surgery; N/A, not available; RHD, rheumatic heart disease; TR, tricuspid regurgitation; TV, tricuspid valve.
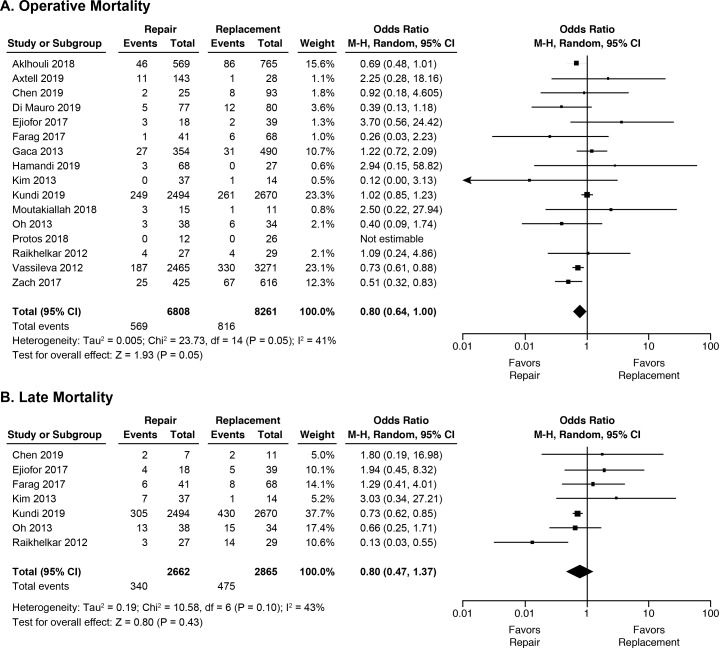
Table 2 displays the pooled analyses for all outcomes of interest. Figure 2 shows the forest plot for mortality. Tricuspid repair was associated with lower rates of operative mortality with borderline statistical significance (8.4% vs 9.9%, OR 0.80, 95% CI 0.64 to 1.00), with no difference in late mortality (12.7% vs 16.6%, OR 0.80, 95% CI 0.47 to 1.37), compared with replacement.
Table 2.
Summary of pooled outcomes of isolated tricuspid repair vs replacement
| Outcome | Studies | N | Repair (%) | Replacement (%) | OR | 95% CI | P value | I2 | χ² (p value) |
| Operative mortality | 16 | 15 069 | 8.4 | 9.9 | 0.80 | 0.64 to 1.00 | 0.05 | 41% | 23.7 (0.05) |
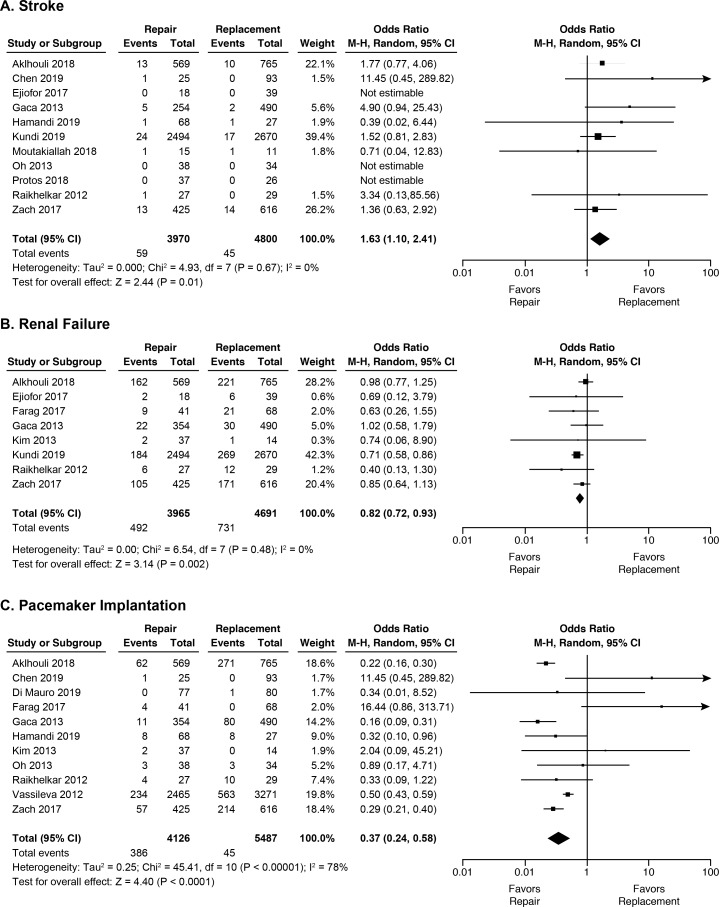
| Stroke | 11 | 8770 | 1.5 | 0.9 | 1.63 | 1.10 to 2.41 | 0.01 | 0% | 4.9 (0.67) |
| Renal failure | 8 | 8656 | 12.4 | 15.6 | 0.82 | 0.72 to 0.93 | 0.002 | 0% | 6.5 (0.48) |
| Prolonged ventilation | 4 | 2350 | 13.8 | 15.2 | 0.90 | 0.62 to 1.30 | 0.56 | 41% | 5.1 (0.17) |
| Mediastinitis | 7 | 6843 | 1.1 | 1.0 | 1.07 | 063 to 1.80 | 0.81 | 4% | 5.2 (0.39) |
| Return to theatre | 6 | 1184 | 11.6 | 14.1 | 0.81 | 0.51 to 1.28 | 0.36 | 9% | 5.5 (0.36) |
| Pacemaker | 11 | 9613 | 9.4 | 21.0 | 0.37 | 0.24 to 0.58 | <0.001 | 78% | 45.4 (<0.001) |
| Late mortality | 7 | 5527 | 12.7 | 16.6 | 0.80 | 0.47 to 1.37 | 0.43 | 43% | 10.6 (0.10) |
Figure 2.
Forrest plots of pooled (A) operative mortality and (B) late mortality for tricuspid valve repair vs replacement.
In terms of postoperative complications, the three with significant differences by surgical technique are shown in figure 3. Tricuspid valve repair was associated with higher rate of stroke (1.5% vs 0.9%, OR 1.63 95% CI 1.10 to 2.41), but lower rates of renal failure (12.4% vs 15.6%, OR 0.82 95% CI (0.72 to 0.93)) and pacemaker implantation (9.4% vs 21.0%, OR 0.37 95% CI (0.24 to 0.58)). Long-term morbidities such as stroke, recurrent tricuspid valve disease, repeat operations and endocarditis were rarely reported and were not pooled.
Figure 3.
Forrest plots of key pooled in-hospital morbidity outcomes (A) stroke, (B) renal failure and (C) pacemaker implantation following tricuspid valve repair vs replacement.
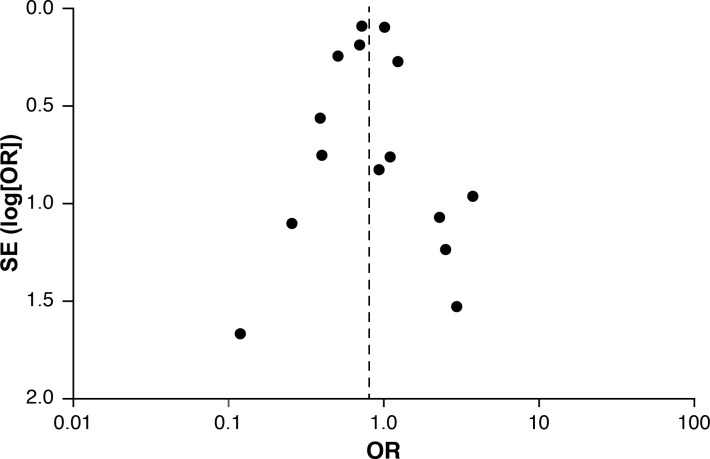
Heterogeneity of studies in reporting adverse events was detected for pacemaker implantation, and to a lesser degree for early and late mortality, prolonged ventilation and return to operating room. Funnel plots were symmetrical without suggesting evidence of publication bias. Figure 4 illustrates the Funnel plot for operative mortality.
Figure 4.
Funnel plot of operative mortality meta-analysis to assess publication bias.
Discussion
This is the first meta-analysis comparing the outcomes of isolated surgical tricuspid valve repair versus replacement. Combining 16 observational studies and over 15 000 patients, we found that isolated tricuspid valve repair was associated with 20% lower operative mortality than replacement, with lower rates of in-hospital acute renal failure and pacemaker implantation, but a higher rate of stroke. There were no differences in other in-hospital complications or late mortality.
Tricuspid repair had lower rates of operative mortality than replacement. Possible reasons from the surgical point of view include preservation of the tricuspid annulus which contributes to right heart function and risk of thromboembolism and bleeding from anticoagulation for prosthetic heart valves. Nevertheless, these patients form a high risk surgical group with 8.4% operative mortality for repair and 9.9% for replacement. Compared with data from the Society of Thoracic Surgeon’s Database, this mortality is much higher versus the 2%–3% mortality for isolated aortic and mitral valve surgeries5 and is more comparable to endocarditis surgery that has a reported risk of 8.2%.14 Possible factors contributing to the high risk of isolated tricuspid valve surgery include high prevalence of previous cardiac surgery, atrial fibrillation, heart failure, right ventricular dilation and/or dysfunction, pulmonary hypertension, non-cardiac comorbidities and late presentation for surgery.6 17 Long-term mortality was less often reported and no differences by type of operation were seen in our analysis.
In terms of in-hospital complications, tricuspid repair had lower rates of renal failure and pacemaker implantation. The former parallels the trend for lower operative mortality and is an important endpoint with prognostic, length of stay, cost and quality of life implications.22 The need for pacemaker is related to the close proximity of the atrioventricular node to the septal leaflet of the tricuspid valve, meaning interventions, manipulation and trauma to the tricuspid valve can lead to heart block, expectedly higher for valve replacement (in fact 21% in our meta-analysis), with underlying heart rhythm disturbance and endocarditis being other important predictors of pacemaker requirement.23 Conduction disturbance needs to be anticipated as transvenous pacemaker lead implantation is not feasible in some types of prosthetic tricuspid valves meaning epicardial leads may be required, sometimes as part of the initial operation. On the other hand, the higher rate of stroke in tricuspid repair was surprising, demonstrating an opposite trend to other endpoints. The reasons for this are unclear. Although prosthetic valves are associated with thromboembolism, in tricuspid valve surgery, this would cause pulmonary emboli unlike left-sided valve replacement, so we would not expect this to contribute to stroke risk. Further studies are required to assess this association and determine potential underlying mechanisms so as to reduce such risk in tricuspid valve repair.
Notably, there was another recent meta-analysis comparing surgical repair versus replacement for tricuspid regurgitation, pooling data from 17 studies totalling 4561 patients.24 Fourteen of the 17 studies included tricuspid valve surgery performed with concomitant other cardiac surgery—in these studies, isolated tricuspid valve surgery made up 0%–41% of the cohort. They similarly found lower rates of all-cause mortality tricuspid repair, but no differences in valve-related events or reoperations. It should be noted that having concurrent left heart surgery with tricuspid valve surgery may often be a lower risk operation than isolated tricuspid valve surgery. For example, a meta-analysis of 15 studies and 2840 patients found tricuspid repair at the time of left-sided heart valve surgery to reduce cardiac mortality and have a trend to reducing all-cause mortality,25 and we know mitral and aortic valve surgeries have lower operative mortality than isolated tricuspid valve surgery.5 Our study adds novel information in being a cleaner cohort focusing only on isolated tricuspid valve surgery and having more than triple the number of patients of this previous meta-analysis.24
Several measures should be applied to improve outcomes for these high risk isolated tricuspid valve surgery patients. The first strategy is to prevent patients from developing severe tricuspid regurgitation, which includes adequately treating underlying causes such as rheumatic heart disease, endocarditis and carcinoid syndrome for primary and left heart disease, chronic lung disease and atrial fibrillation for secondary tricuspid valve disease. An aggressive approach to operating on the tricuspid valve even at just mild or moderate severity at time of left heart surgery as recommended by guidelines should be undertaken,1 4 given the improvements in clinical outcomes and reductions in late tricuspid regurgitation without added operative risk reported in other studies.25 26 Second, it may be that patients are often monitored for a prolonged period of time due to the reluctance to refer for surgery and earlier surgery than what is suggested in the guidelines may need to be considered and investigated, taking into account clinical (such as frailty), biomarker (such as B-type natriuretic peptide) and multi-modality imaging (such as accurate right ventricular volume, function and strain analysis) parameters.1 4 Third, accurate risk stratification is necessary to improve patient selection for surgery. Further evaluations and refinements on existing surgical risk models are necessary, as currently only one is designed specifically for tricuspid valve surgery, and this is without external validation.27 Fourth, the rates of surgical repair at 45% in this meta-analysis may need to be increased where possible, and these patients should be funnelled towards experienced cardiac surgeons and even centres of excellence. Finally, there needs to be thorough attention to optimising these patients’ perioperative status and management, such as diuretics for improved fluid status, cardioplegia strategies to reduce ischaemic time and prevention of in-hospital complications.
Other treatment strategies need to be sought to improve the outcomes of patients with isolated severe tricuspid valve disease. The role of surgery for isolated tricuspid regurgitation was questioned in a recent study also included in this meta-analysis. In this study, although surgery yielded higher survival in the overall cohort, in the propensity-matched subgroups, surgery did not improve outcomes over conservative medical therapy.9 This analysis was limited by power (124 patients total), but further questions the timing of surgery, especially when patients are high risk for surgery because of underlying clinical status and/or comorbidities. It is in this scenario where transcatheter tricuspid valve interventions may play an important role in the future. The current experience is limited compared with aortic or mitral valve interventions but appears promising with low 30 day mortality of 3.6% and high 1-year survival, although the procedural success rate of 73% still needs to be improved.3 Furthermore in a subsequent propensity-matched observational study, percutaneous tricuspid interventions had higher survival and freedom from heart failure rehospitalisations compared with medical therapy alone. Randomised trials for different tricuspid devices are necessary and are currently in planning or in progress and will allow further understanding of the role of this promising intervention in clinical practice.28
This meta-analysis has some limitations. There are no randomised trials comparing tricuspid valve repair and replacement; all studies were observational and thus susceptible to error with regard to patient selection and characteristics. Such differences in baseline characteristic are expected, but were inconsistently reported and patient-level data were not available for us to perform multivariable analyses such as meta-regression to identify important predictors of adverse outcomes. These include in particular aetiology of tricuspid valve disease, left and right heart function and operative strategy including specific repair techniques or prosthetic valves used. Certainly, inability to repair may be a marker for a higher risk patient, such as in endocarditis with significant destruction of the tricuspid valve. Heterogeneity existed in the study design and outcomes recorded by studies as well. Analysis of less frequent events was likely underpowered. Insufficient studies reported long-term morbidity outcomes such as stroke, endocarditis, repeat tricuspid valve operations for recurrent valvular dysfunction, symptoms and quality of life for pooled analyses which are all important endpoints to consider. Publication bias although not found may be present as a common problem for all meta-analyses.
In conclusion, this meta-analysis found that isolated tricuspid valve repair was associated with decreased rates of operative mortality (of borderline significance), renal failure and pacemaker implantation, so surgical repair should generally be recommended when feasible, similar to mitral valve surgery. The higher pooled stroke rates following tricuspid valve repair was unexpected, and warrants further research. The operative risk of both procedures remains high, and further investigations and experience with regard to earlier surgery, percutaneous interventions and medical therapy are necessary to try and improve outcomes in this challenging group of patients.
Footnotes
Contributors: TKMW and MYD were involved with planning, literature search, article screening, data extraction, statistical analysis, data interpretation, writing and final approval of the manuscript. BG, RM, BX, ZBP, GP and AMG were involved in the planning, data interpretation, revision and final approval of the manuscript.
Funding: TKMW is supported by the Overseas Clinical and Research Fellowship (grant number 1775) from the National Heart Foundation of New Zealand. MYD is supported by the Haslam Family endowed chair in cardiovascular medicine at Cleveland Clinic.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1.Baumgartner H, Falk V, Bax JJ, et al. . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 2.Sadeghpour A, Hassanzadeh M, Kyavar M, et al. . Impact of severe tricuspid regurgitation on long term survival. Res Cardiovasc Med 2013;2:121–6. 10.5812/cardiovascmed.10686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taramasso M, Alessandrini H, Latib A, et al. . Outcomes after current transcatheter tricuspid valve intervention: mid-term results from the International TriValve registry. JACC Cardiovasc Interv 2019;12:155–65. 10.1016/j.jcin.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association Task force on practice guidelines. J Am Coll Cardiol 2014;63:e57–185. 10.1016/j.jacc.2014.02.536 [DOI] [PubMed] [Google Scholar]
- 5.O'Brien SM, Feng L, He X, et al. . The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-Statistical Methods and Results. Ann Thorac Surg 2018;105:1419–28. 10.1016/j.athoracsur.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Zack CJ, Fender EA, Chandrashekar P, et al. . National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 2017;70:2953–60. 10.1016/j.jacc.2017.10.039 [DOI] [PubMed] [Google Scholar]
- 7.Vassileva CM, Shabosky J, Boley T, et al. . Tricuspid valve surgery: the past 10 years from the nationwide inpatient sample (NIS) database. J Thorac Cardiovasc Surg 2012;143:1043–9. 10.1016/j.jtcvs.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 8.Alkhouli M, Berzingi C, Kowatli A, et al. . Comparative early outcomes of tricuspid valve repair versus replacement for secondary tricuspid regurgitation. Open Heart 2018;5:e000878 10.1136/openhrt-2018-000878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axtell AL, Bhambhani V, Moonsamy P, et al. . Surgery Does Not Improve Survival in Patients With Isolated Severe Tricuspid Regurgitation. J Am Coll Cardiol 2019;74:715–25. 10.1016/j.jacc.2019.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Hu K, Ma W, et al. . Isolated reoperation for tricuspid regurgitation after left-sided valve surgery: technique evolution. Eur J Cardiothorac Surg 2020;57:142–50. 10.1093/ejcts/ezz160 [DOI] [PubMed] [Google Scholar]
- 11.Di Mauro M, Foschi M, Dato GMA, et al. . Surgical treatment of isolated tricuspid valve infective endocarditis: 25-year results from a multicenter registry. Int J Cardiol 2019;292:62–7. 10.1016/j.ijcard.2019.05.020 [DOI] [PubMed] [Google Scholar]
- 12.Ejiofor JI, Neely RC, Yammine M, et al. . Surgical outcomes of isolated tricuspid valve procedures: repair versus replacement. Ann Cardiothorac Surg 2017;6:214–22. 10.21037/acs.2017.05.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farag M, Arif R, Sabashnikov A, et al. . Repair or replacement for isolated tricuspid valve pathology? insights from a surgical analysis on long-term survival. Med Sci Monit 2017;23:1017–25. 10.12659/MSM.900841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaca JG, Sheng S, Daneshmand M, et al. . Current outcomes for tricuspid valve infective endocarditis surgery in North America. Ann Thorac Surg 2013;96:1374–81. 10.1016/j.athoracsur.2013.05.046 [DOI] [PubMed] [Google Scholar]
- 15.Hamandi M, Smith RL, Ryan WH, et al. . Outcomes of isolated tricuspid valve surgery have improved in the modern era. Ann Thorac Surg 2019;108:11–15. 10.1016/j.athoracsur.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 16.Kim JB, Jung S-H, Choo SJ, et al. . Clinical and echocardiographic outcomes after surgery for severe isolated tricuspid regurgitation. J Thorac Cardiovasc Surg 2013;146:278–84. 10.1016/j.jtcvs.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 17.Kundi H, Popma JJ, Cohen DJ, et al. . Prevalence and outcomes of isolated tricuspid valve surgery among Medicare beneficiaries. Am J Cardiol 2019;123:132–8. 10.1016/j.amjcard.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 18.Moutakiallah Y, Aithoussa M, Atmani N, et al. . Reoperation for isolated rheumatic tricuspid regurgitation. J Cardiothorac Surg 2018;13:104 10.1186/s13019-018-0793-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh TH, Wang TK, Sidhu K, et al. . Isolated tricuspid valve surgery at a single centre: the 47-year Auckland experience, 1965-2011. Interact Cardiovasc Thorac Surg 2014;18:27–32. 10.1093/icvts/ivt452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Protos AN, Trivedi JR, Whited WM, et al. . Valvectomy Versus Replacement for the Surgical Treatment of Tricuspid Endocarditis. Ann Thorac Surg 2018;106:664–9. 10.1016/j.athoracsur.2018.04.051 [DOI] [PubMed] [Google Scholar]
- 21.Raikhelkar J, Lin H-M, Neckman D, et al. . Isolated tricuspid valve surgery: predictors of adverse outcome and survival. Heart Lung Circ 2013;22:211–20. 10.1016/j.hlc.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 22.O'Neal JB, Shaw AD, Billings FT, FTt B. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care 2016;20:187 10.1186/s13054-016-1352-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann FEM, Graf H, Wellmann P, et al. . Etiology of tricuspid valve disease is a predictor of bradyarrhythmia after tricuspid valve surgery. J Cardiovasc Electrophysiol 2019;30:1108–16. 10.1111/jce.13937 [DOI] [PubMed] [Google Scholar]
- 24.Choi JW, Jang M-J, Kim KH, et al. . Repair versus replacement for the surgical correction of tricuspid regurgitation: a meta-analysis. Eur J Cardiothorac Surg 2018;53:748–55. 10.1093/ejcts/ezx420 [DOI] [PubMed] [Google Scholar]
- 25.Pagnesi M, Montalto C, Mangieri A, et al. . Tricuspid annuloplasty versus a conservative approach in patients with functional tricuspid regurgitation undergoing left-sided heart valve surgery: a study-level meta-analysis. Int J Cardiol 2017;240:138–44. 10.1016/j.ijcard.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 26.Pettinari M, De Kerchove L, Lazam S, et al. . Mid-Term results of a randomized trial of tricuspid annuloplasty for less-than-severe functional tricuspid regurgitation at the time of mitral valve surgery†. Eur J Cardiothorac Surg 2019;55:851–8. 10.1093/ejcts/ezy378 [DOI] [PubMed] [Google Scholar]
- 27.LaPar DJ, Likosky DS, Zhang M, et al. . Development of a risk prediction model and clinical risk score for isolated tricuspid valve surgery. Ann Thorac Surg 2018;106:129–36. 10.1016/j.athoracsur.2017.11.077 [DOI] [PubMed] [Google Scholar]
- 28.Taramasso M, Benfari G, van der Bijl P, et al. . Transcatheter Versus Medical Treatment of Patients With Symptomatic Severe Tricuspid Regurgitation. J Am Coll Cardiol 2019;74:2998–3008. 10.1016/j.jacc.2019.09.028 [DOI] [PubMed] [Google Scholar]