Figure 2.
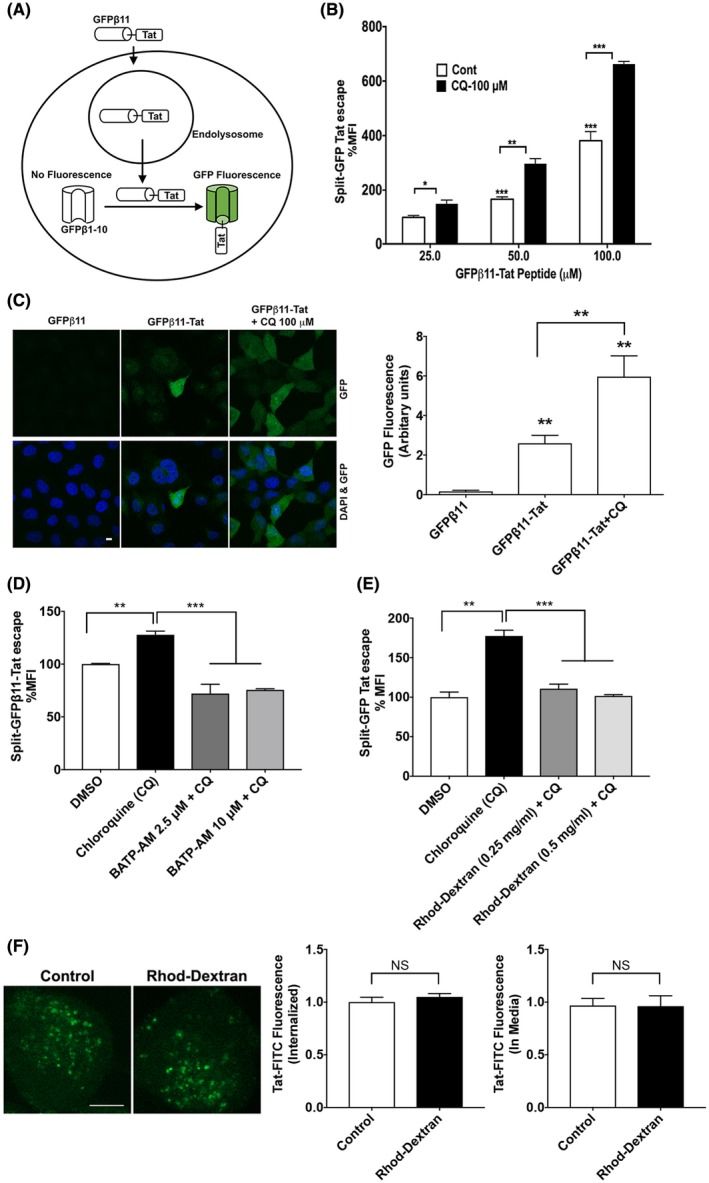
Calcium is involved in Tat endolysosome escape. A, A split‐GFP fluorescence assay was used to assess Tat endolysosome escape in H1299 cells stably expressing fluorescence complementation of intracellularly GFPβ1‐10 protein fragment. Following endolysosome escape, extracellular addition of GFPβ11‐Tat peptide induces the completion of GFP fluorescence in a concentration‐dependent manner. B, Flow cytometry data show that chloroquine (100 μM) dramatically enhanced Tat endolysosome escape (n = 3; *P < .05; **P < .01; ***P < .001). C, Confocal imaging shows that GFPβ11‐Tat (50 μM), but not GFPβ11 (50 μM) significantly induced GFP fluorescence, and chloroquine (100 μM) enhanced GFPβ11‐Tat‐induced GFP fluorescence (scale bar = 10 μm) (n = 5; **P < .01). D, Chelating cytosolic calcium with BAPTA‐AM (2.5‐10 μM) significantly decreased Tat‐endolysosome escape (n = 3; **P < .01; ***P < .001). E, Chelating endolysosome calcium with high‐affinity rhodamine‐dextran (0.5 mg/mL) significantly prevented chloroquine‐induced increases in Tat‐mediated LTR transactivation (n = 3; *P < .05). F, Chelating endolysosome calcium with high‐affinity rhodamine‐dextran (0.5 mg/mL) did not affect the internalization of Tat‐FITC (n = 4, P > .05, bar = 10 μm)
